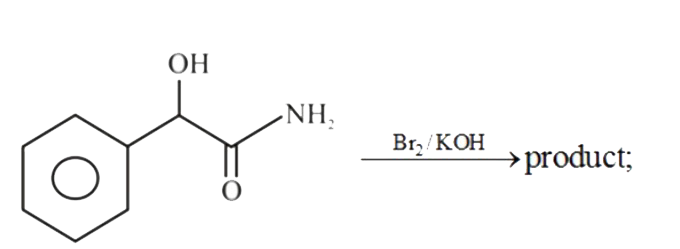
Give the IUPAC name and structure of the amine obtained when 3-chlorobutanamide undergoes Hoffmann- bromamide reaction.
To solve the problem, we need to determine the IUPAC name and structure of the amine obtained when 3-chlorobutanamide undergoes the Hofmann bromamide reaction.
1. Identify the Starting Compound: The starting compound is 3-chlorobutanamide. The structure of 3-chlorobutanamide can be represented as:
- CH3-CH(Cl)-CH2-CO-NH2
- Here, the amide functional group (–CO-NH2) is attached to the butane chain at the end of the carbon chain.
2. Understand the Hofmann Bromamide Reaction: The Hofmann bromamide reaction involves the conversion of an amide to a primary amine with the loss of one carbon atom. This reaction typically involves the treatment of the amide with bromine (Br2) in the presence of a strong base (like NaOH).
3. Determine the Product Structure: In this case, when 3-chlorobutanamide undergoes the Hofmann bromamide reaction, one carbon atom is removed from the carbon chain. The carbon that is removed is the carbonyl carbon (C=O) of the amide group. Thus, the remaining structure will have three carbons:
- The structure after the reaction will be CH3-CH(Cl)-CH2-NH2.
4. Identify the New IUPAC Name: The resulting compound from the reaction is a primary amine. The carbon chain has three carbons, and there is a chlorine substituent on the second carbon. Therefore, the IUPAC name of the product is 2-chloropropan-1-amine.
5. Final Structure Representation: The final structure of the amine can be drawn as follows:
- CH3-CH(Cl)-CH2-NH2
Final Answer:
- IUPAC Name: 2-chloropropan-1-amine
- Structure:
Cl∣CH3−CH−CH2−NH2
- CH3-CH(Cl)-CH2-CO-NH2
- Here, the amide functional group (–CO-NH2) is attached to the butane chain at the end of the carbon chain.
- The structure after the reaction will be CH3-CH(Cl)-CH2-NH2.
- CH3-CH(Cl)-CH2-NH2
- IUPAC Name: 2-chloropropan-1-amine
- Structure:
Cl∣CH3−CH−CH2−NH2
Similar Questions
Knowledge Check
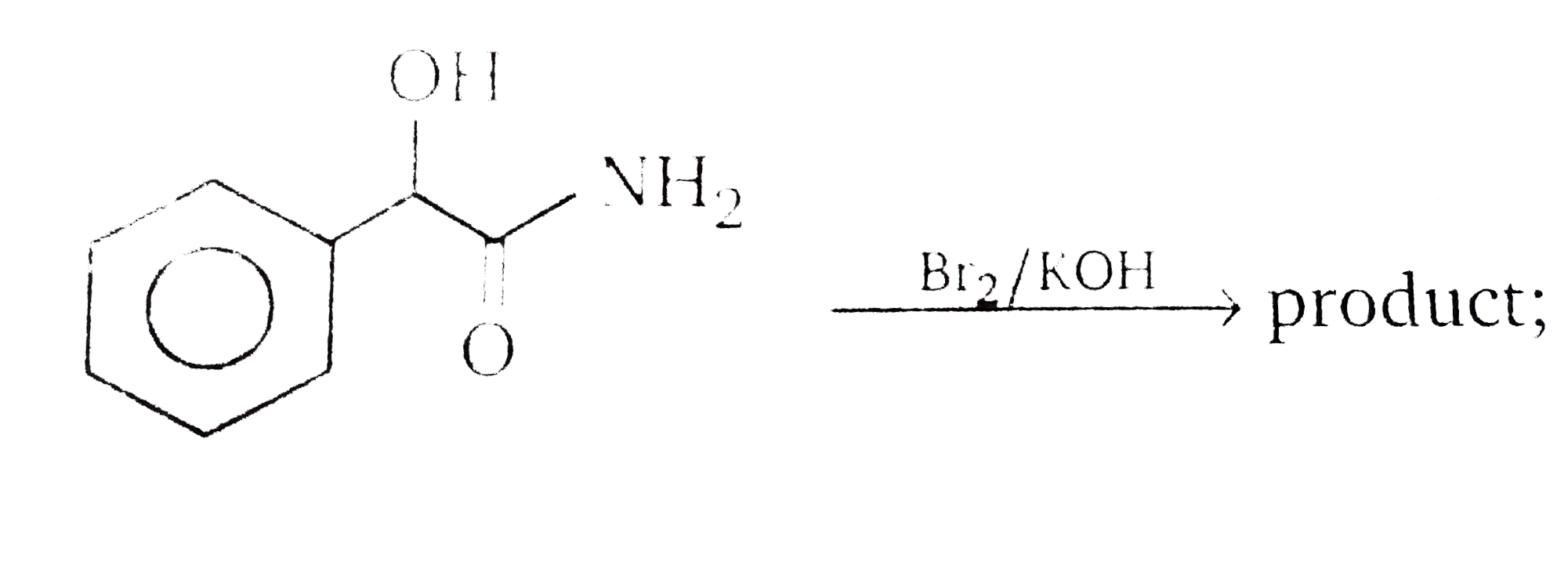
Product of this Hoffmann bromamide reaction is :APh−O∣∣C−CH3BPh - CHOC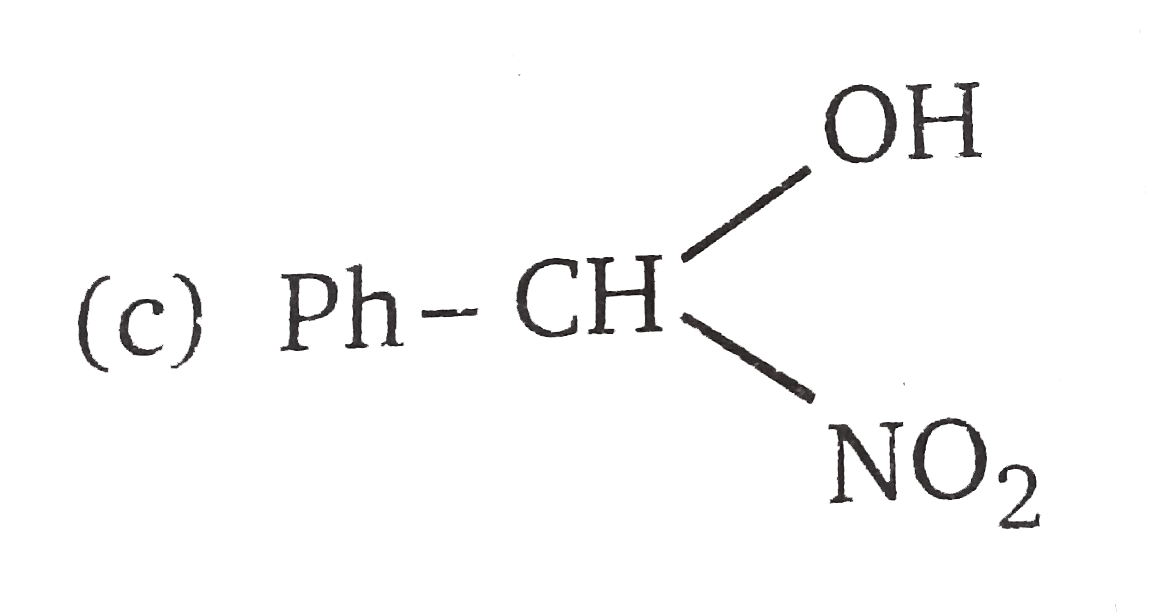 DPh−CH2−NH2
DPh−CH2−NH2Which of the following amides will not undergo Hofmann bromamide reaction?
ACH3CONHCH3BCH3CH2CONH2CCH3CONH2DC6H5CONH2Which of the following amides will not undergo Hofmann bromamide reaction ?
AEthanamideBPropanamideCBenzenamideDAcetanilide
Hoffmann Bromamide Degradation Reaction
View SolutionGive the IUPAC name of the following structure
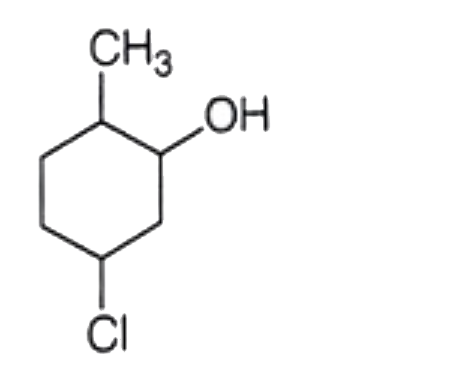
View SolutionHow can you convert an amide into an amine having one caron less than the starting compound ?
(b) Name the reaction.
(c) Give the IUPAC name and structure of the amine obtained by the above method if the amide is 3-chlorobutanamide.
View SolutionWhich of the following amides will not undergo Hofmann bromamide reaction?
View SolutionThe IUPAC name of ethyl methyl amine is
View Solution