Define the term 'order of reaction' for chemical reactions.
Video Solution
Text Solution
The order of a reaction is equal to the sum of the powers (exponents) to which the various concentration terms are raised in the rate law expression of the reaction.
|
Similar Questions
Knowledge Check
The chemical reaction,
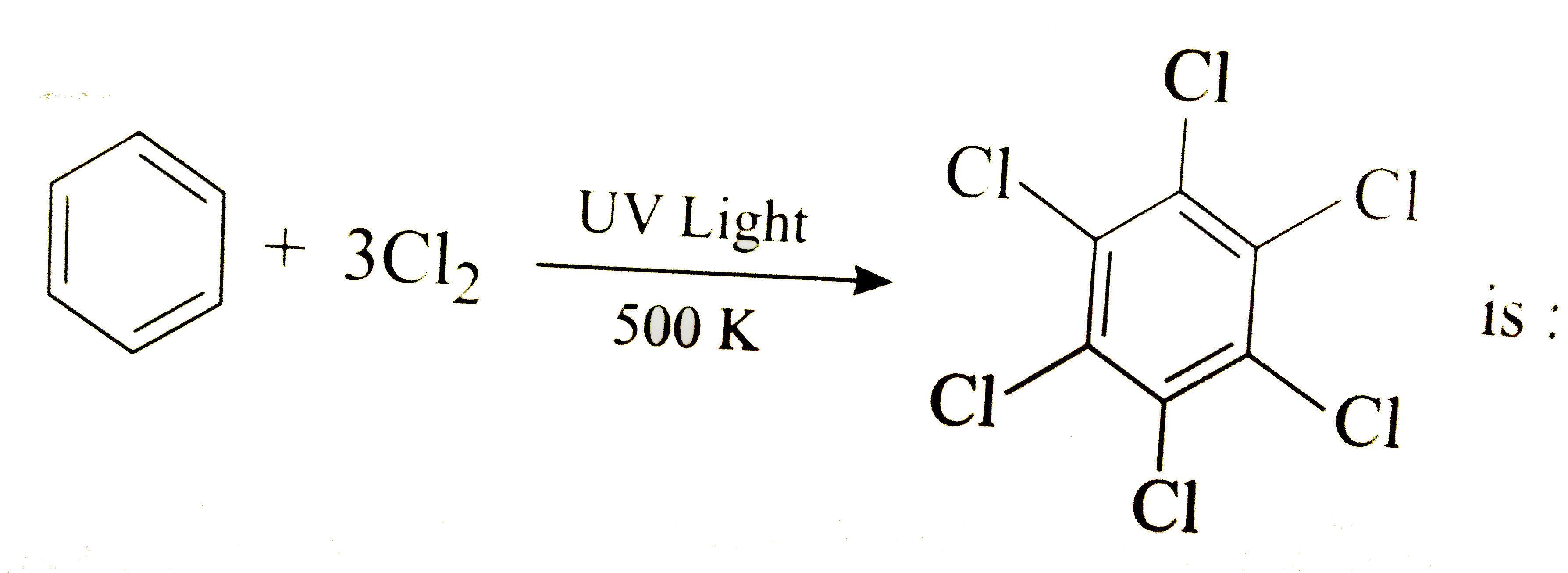 Aan addition reactionBan elimination reactionCa substitution reactionDrearrengement reaction
Aan addition reactionBan elimination reactionCa substitution reactionDrearrengement reactionWhen the rate of reaction is independent of the concentration of reactant then the order of that chemical reaction is
AoneBtwoCthreeDzero
Define the term Chemical reaction
View SolutionDefine 'order of a reaction'.
View SolutionDefine 'order of a reaction'.
View SolutionOrder of a chemical reaction.
View SolutionFor a Ist order chemical reaction,
View SolutionWrite the formula representation of rate of chemical reaction. Define.
View Solution

