i. Give a chemical test to distinguish between N-methylethanamine and N,N-dimethyl ethanamine.
ii. Write the reaction for catalytic reduction of nitrobenzene followed by reaction of product so formed with bromine water.
iii. Out of butan-1-ol and butan-1-amine, which will be more soluble in water and why?
(i) N-methyletahnamine is a secondary amine. When it reacts with benzenesulphonyl chloride, it forms N- Ethyl -N methyl sulphonamide while and N,N-dimethyl etahnanmine is a tertiary amine it does not react with benzenesulphonyl chloride.
(ii) 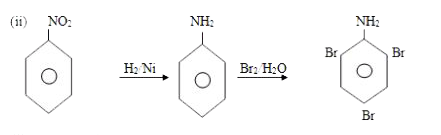
(iii) Butan-1-ol Alcohol forms stronger hydrogen bonds with water than formed by amine due to higher electronegativity of O in alcohol than N in amine
Similar Questions
(a) Write coupling reaction.
(b) Give a chemical test to distinguish between aniline and N-methylaniline.
(c) How will you convert benzoic acid to aniline?
View SolutionRead the given passage and answer questions number 1 to 5 that follow :
The lower aliphatic amines are gases with fishy odour. Primary amines with three or more carbon atoms are liquid and still higher one are solid. Aniline and other arylamines are usually colourless but get coloured on storage due to atmospheric oxidation. Lower aliphatic amines are soluble in water because they can form hydrogen bonds with water molecules. However, solubility decreases with increase in molar mass of amines due to increase in size of the hydrophobic alkyl part. Higher amines are essentially insoluble in water. Considering the electronegativity of nitrogen of amine and oxygen of alcohols in water. Out of butan-1-ol and butan-1-amine, which will be more soluble in water and why? Amines are soluble in organic solvents like alcohol, ether and benzene. You may remember that alcohols are more polar than amines and form stronger intermolecular hydrogen bonds than amines. Primary and secondary amines are engaged in intermolecular association due to hydrogen bonding between nitrogen of one and hydrogen of another molecule. This intermolecular association is more in primary amines than in secondary amines as there are two hydrogen atoms available for hydrogen bond formation in it. Tertiary amines do not have intermolecular association due to the absence of hydrogen atom available for hydrogen bond formation. Therefore, the order of boiling points of isomeric amines is as follows :
Primary > Secondary > Tertiary.
Out of C5H11OH and C5H11NH2, which is more soluble in water and why ?
View SolutionRead the given passage and answer questions number 1 to 5 that follow :
The lower aliphatic amines are gases with fishy odour. Primary amines with three or more carbon atoms are liquid and still higher one are solid. Aniline and other arylamines are usually colourless but get coloured on storage due to atmospheric oxidation. Lower aliphatic amines are soluble in water because they can form hydrogen bonds with water molecules. However, solubility decreases with increase in molar mass of amines due to increase in size of the hydrophobic alkyl part. Higher amines are essentially insoluble in water. Considering the electronegativity of nitrogen of amine and oxygen of alcohols in water. Out of butan-1-ol and butan-1-amine, which will be more soluble in water and why? Amines are soluble in organic solvents like alcohol, ether and benzene. You may remember that alcohols are more polar than amines and form stronger intermolecular hydrogen bonds than amines. Primary and secondary amines are engaged in intermolecular association due to hydrogen bonding between nitrogen of one and hydrogen of another molecule. This intermolecular association is more in primary amines than in secondary amines as there are two hydrogen atoms available for hydrogen bond formation in it. Tertiary amines do not have intermolecular association due to the absence of hydrogen atom available for hydrogen bond formation. Therefore, the order of boiling points of isomeric amines is as follows :
Primary > Secondary > Tertiary.
Out of benzene and water which will dissolve C6H13NH2 more ?
View SolutionRead the given passage and answer questions number 1 to 5 that follow :
The lower aliphatic amines are gases with fishy odour. Primary amines with three or more carbon atoms are liquid and still higher one are solid. Aniline and other arylamines are usually colourless but get coloured on storage due to atmospheric oxidation. Lower aliphatic amines are soluble in water because they can form hydrogen bonds with water molecules. However, solubility decreases with increase in molar mass of amines due to increase in size of the hydrophobic alkyl part. Higher amines are essentially insoluble in water. Considering the electronegativity of nitrogen of amine and oxygen of alcohols in water. Out of butan-1-ol and butan-1-amine, which will be more soluble in water and why? Amines are soluble in organic solvents like alcohol, ether and benzene. You may remember that alcohols are more polar than amines and form stronger intermolecular hydrogen bonds than amines. Primary and secondary amines are engaged in intermolecular association due to hydrogen bonding between nitrogen of one and hydrogen of another molecule. This intermolecular association is more in primary amines than in secondary amines as there are two hydrogen atoms available for hydrogen bond formation in it. Tertiary amines do not have intermolecular association due to the absence of hydrogen atom available for hydrogen bond formation. Therefore, the order of boiling points of isomeric amines is as follows :
Primary > Secondary > Tertiary.
Why does the solubility of amines decrease with the increase of molar mass ?
View SolutionRead the given passage and answer questions number 1 to 5 that follow :
The lower aliphatic amines are gases with fishy odour. Primary amines with three or more carbon atoms are liquid and still higher one are solid. Aniline and other arylamines are usually colourless but get coloured on storage due to atmospheric oxidation. Lower aliphatic amines are soluble in water because they can form hydrogen bonds with water molecules. However, solubility decreases with increase in molar mass of amines due to increase in size of the hydrophobic alkyl part. Higher amines are essentially insoluble in water. Considering the electronegativity of nitrogen of amine and oxygen of alcohols in water. Out of butan-1-ol and butan-1-amine, which will be more soluble in water and why? Amines are soluble in organic solvents like alcohol, ether and benzene. You may remember that alcohols are more polar than amines and form stronger intermolecular hydrogen bonds than amines. Primary and secondary amines are engaged in intermolecular association due to hydrogen bonding between nitrogen of one and hydrogen of another molecule. This intermolecular association is more in primary amines than in secondary amines as there are two hydrogen atoms available for hydrogen bond formation in it. Tertiary amines do not have intermolecular association due to the absence of hydrogen atom available for hydrogen bond formation. Therefore, the order of boiling points of isomeric amines is as follows :
Primary > Secondary > Tertiary.
Arrange the following compounds in increasing order of boiling point :
CH3−CH2−CH∣CH3−NH2,CH3−CH3∣C∣CH3−NH2,CH3CH2CH2CH2NH2
View Solution(a) Give a chemical test to distinguish between saturated and unsaturated hydrocarbon.
(b) Name the products formed when ethane burns in air. Write the balanced chemical equatioin for the reaction showing the types of energies liberated.
(c) Why is reactiion between methane and chlorine in the presence of sunlight considered a substitution reaction ?
View Solution(a) Give a chemical test to distinguish between saturated and unsaturated hydrocarbon.
(b) Name the products formed when ethane burns in air. Write the balanced chemical equation for the reaction showing the types of energies liberated.
(c) Why is reaction between methane and chlorine in the presence of sunlight considered a substitution reaction ?
View Solution(a) Give one chemical test to distinguish between the following pairs of compounds :
(i) Methylamine and dimethylamine (ii) Aniline and benzylamine.
(b) Write the structures of different isomers corresponding to the molecular formula C3H9N, which will liberate nitrogen gas on treatment with nitrous acid.
View SolutionGive one chemical test to distinguish between the following pairs of compounds .
i. Methylamine and dimethylamine
ii. Secondary and tertiary amines
iii. Ethylamine and aniline
iv. Aniline and benzylamine
v. Aniline and N-methylaniline
View Solution(a) Give chemical tests to distinguish between
(i) Phenol and Benzoic acid
(ii) Benzophenone and Acetophenone
(b) Write the strucutre of the main products of following reactions :
(i)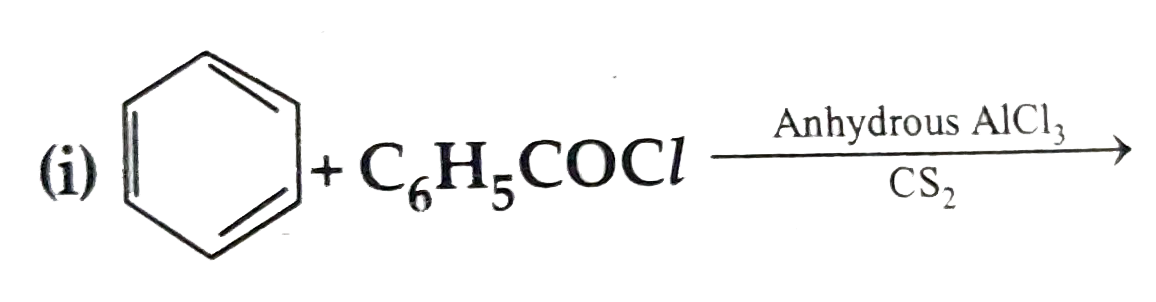
(ii) H3C−C≡C−HHg2+,H2SO4−−−−−−−−→
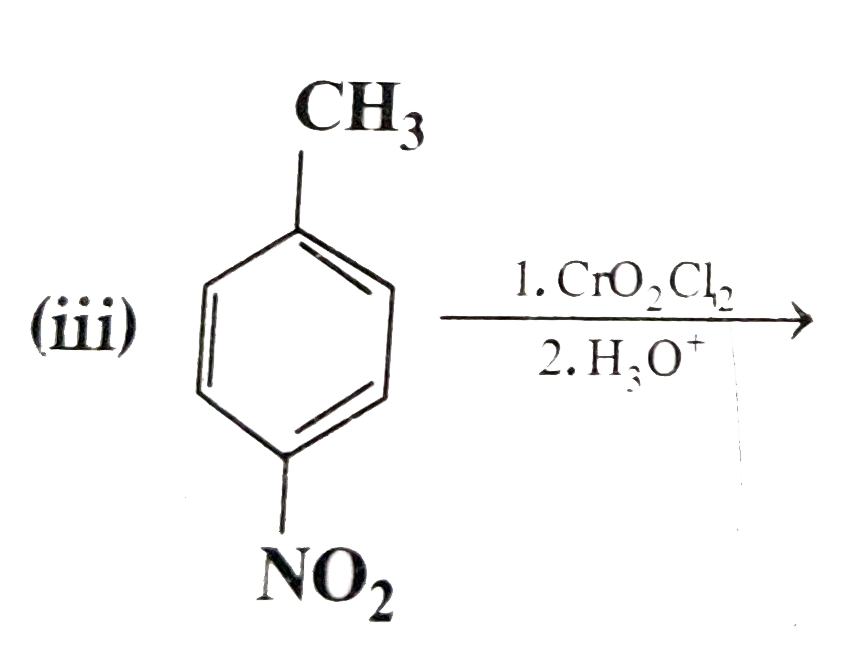 .
.
View Solution
