Which of the following are correct statements ?
A
Spontaneous adsorption of gases on solid surface is an exothermic process as entropy decreases during adsorption
B
Formation of micelles takes place when temperature is below Kraft Temperature (Tk) and concentration is above critical micelle concentration (CMC)
C
Longer the length of hydrophobic chain, smaller is the value of critical micelle concentration (CMC)
D
According to Hardy-Schulze rule the coagulation (flocculating) value of Fe3+ ion will be more than Ba2+ or Na+.
Text Solution
The correct Answer is:A, C
|
Topper's Solved these Questions
- SURFACE CHEMISTRYBOOK - RESONANCECHAPTER - SURFACE CHEMISTRYEXERCISE - Exercise-2, Part-IV Comprehension
9 Videos
- SURFACE CHEMISTRYBOOK - RESONANCECHAPTER - SURFACE CHEMISTRYEXERCISE - Exercise-3, Part-I JEE (Advanced)
13 Videos
- SURFACE CHEMISTRYBOOK - RESONANCECHAPTER - SURFACE CHEMISTRYEXERCISE - Exercise-2, Part-II
7 Videos
- STRUCTURAL IDENTIFICATIONBOOK - RESONANCECHAPTER - STRUCTURAL IDENTIFICATIONEXERCISE - Advanced level Problems (Part-III)
12 Videos
- TEST PAPERSBOOK - RESONANCECHAPTER - TEST PAPERSEXERCISE - PT-03
1 Video
Similar Questions
Knowledge Check
Which of the following are correct statement regarding these molecules.
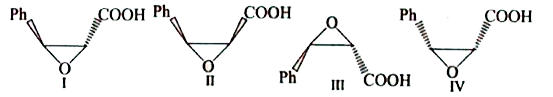 ACompound I and II are diastereomersBCompound II and IV are enantiomersCCompound I and III are enantiomersDCompound III and IV are homomers
ACompound I and II are diastereomersBCompound II and IV are enantiomersCCompound I and III are enantiomersDCompound III and IV are homomersWhich of the following are correct statement regarding these molecules.
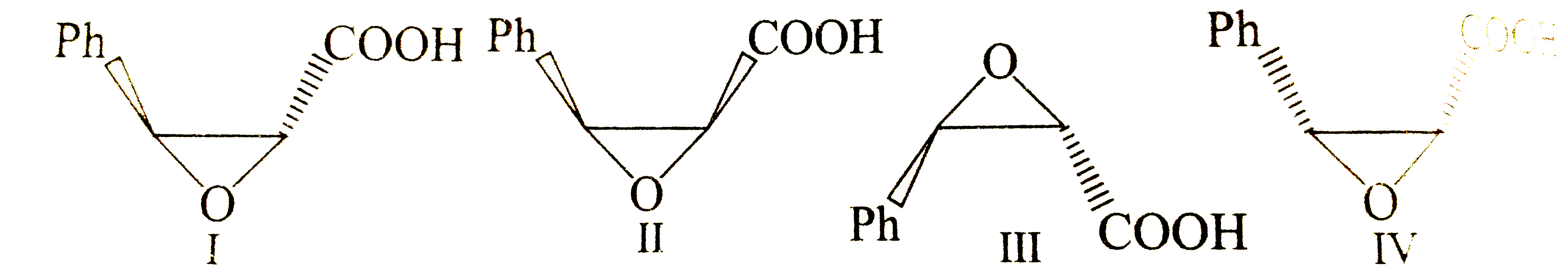 ACompound I and II are diastereomersBCompound II and IV are enantiomersCCompound I and III are enantimersDCompound III and IV are homomers
ACompound I and II are diastereomersBCompound II and IV are enantiomersCCompound I and III are enantimersDCompound III and IV are homomersWhich of the following is a correct statement ?
Aβ-rays are same as cathode raysBGamma rays are high energy electronsCAlpha particles are singly ionised helium atomsDProtons and neutrons have exactly the same mass

