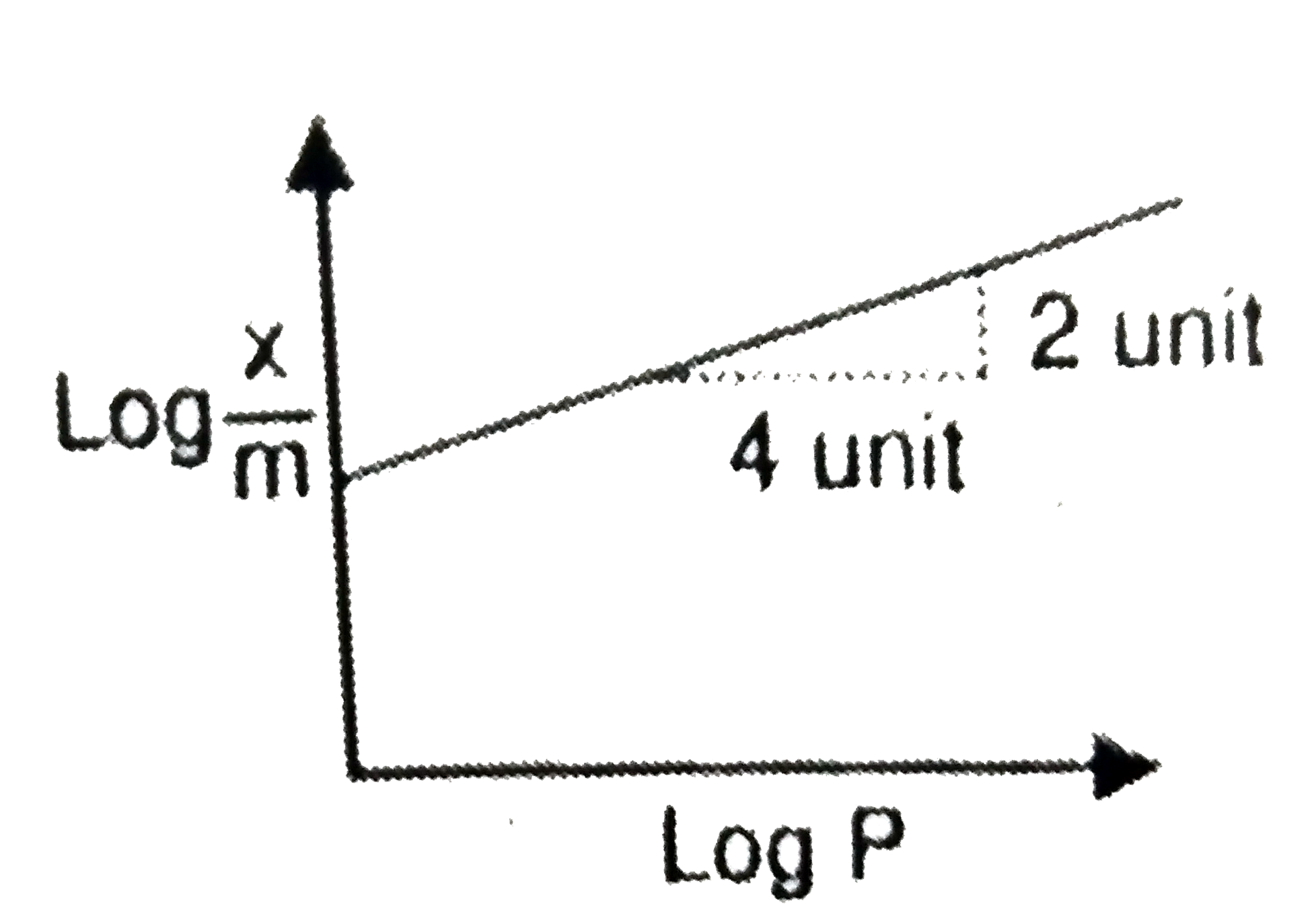
Adsorption of a gas follows Freundlich adsorption isotherm. In the given plot, x is the mass of the gas adsorbed on mass m of the adsorbent at pressure p. xm is proportional to :
p2
p
p1/2
p1/4
The correct Answer is:C
Similar Questions
If a gas absorbs 200J of heat and expands by 500cm3 against a constant pressure of 2×105Nm−2, then the change in internal energy is
View SolutionAdsorption of gas follows Freundlich adsorption isotherm. If x is the mass of the gas adsorbed on mass m of the adsrobent , the correct plot of xm versus p is :
View Solution
Knowledge Check
Adsorption of gas follows Freundlich adsorption isotherm. If x is the mass of the gas adsorbed on mass m of the adsrobent , the correct plot of xm versus p is :
A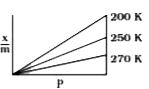 B
B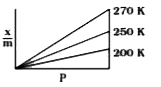 C
C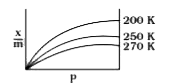 D
D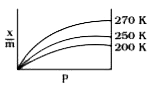
Adsorption of a gas follows Freundlich adsorption isotherm. x is the mass of the gas adsorbed on mass m of the adsorbent. The plot of logxm versus log p is shown in the graph. xm is proportional to:
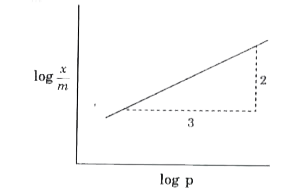 Ap2/3Bp3/2Cp3Dp2
Ap2/3Bp3/2Cp3Dp2Adsorptions of gas follows Freundich adsorption isotherm . X is the mass of the gas adsorbed on mass m of the adsorbent. The plot of logxm versus log p is logxm shown in the given graph xm is propotional to:
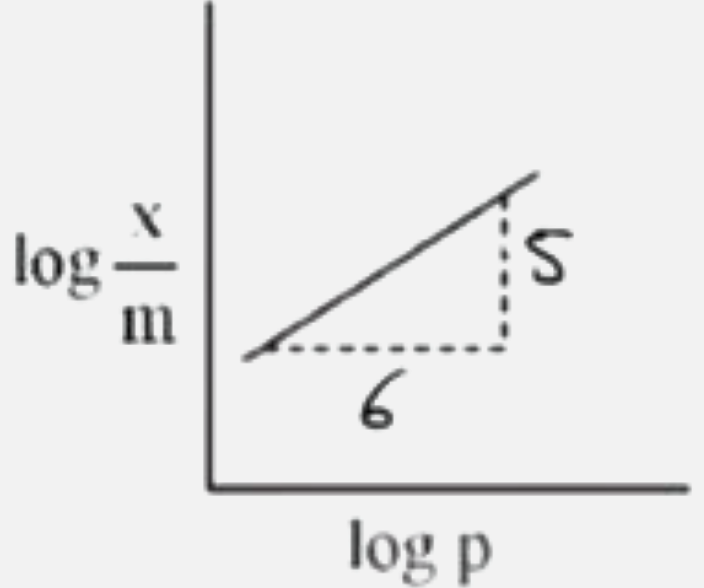 AP6BP6/5CP5DP5/6
AP6BP6/5CP5DP5/6
Adsorption of gas follows Freundlich adsorption isotherm. If x is the mass of the gas adsorbed on mass m of the adsrobent , the correct plot of xm versus p is :
View SolutionAdsorptionof a gas follows Freundlich adsorption isotherm. x is the mass of the gas adsorbed on mass m of the adsorbent.
The plot of logxm versus log p is shown in the given graph xm is proportional to :
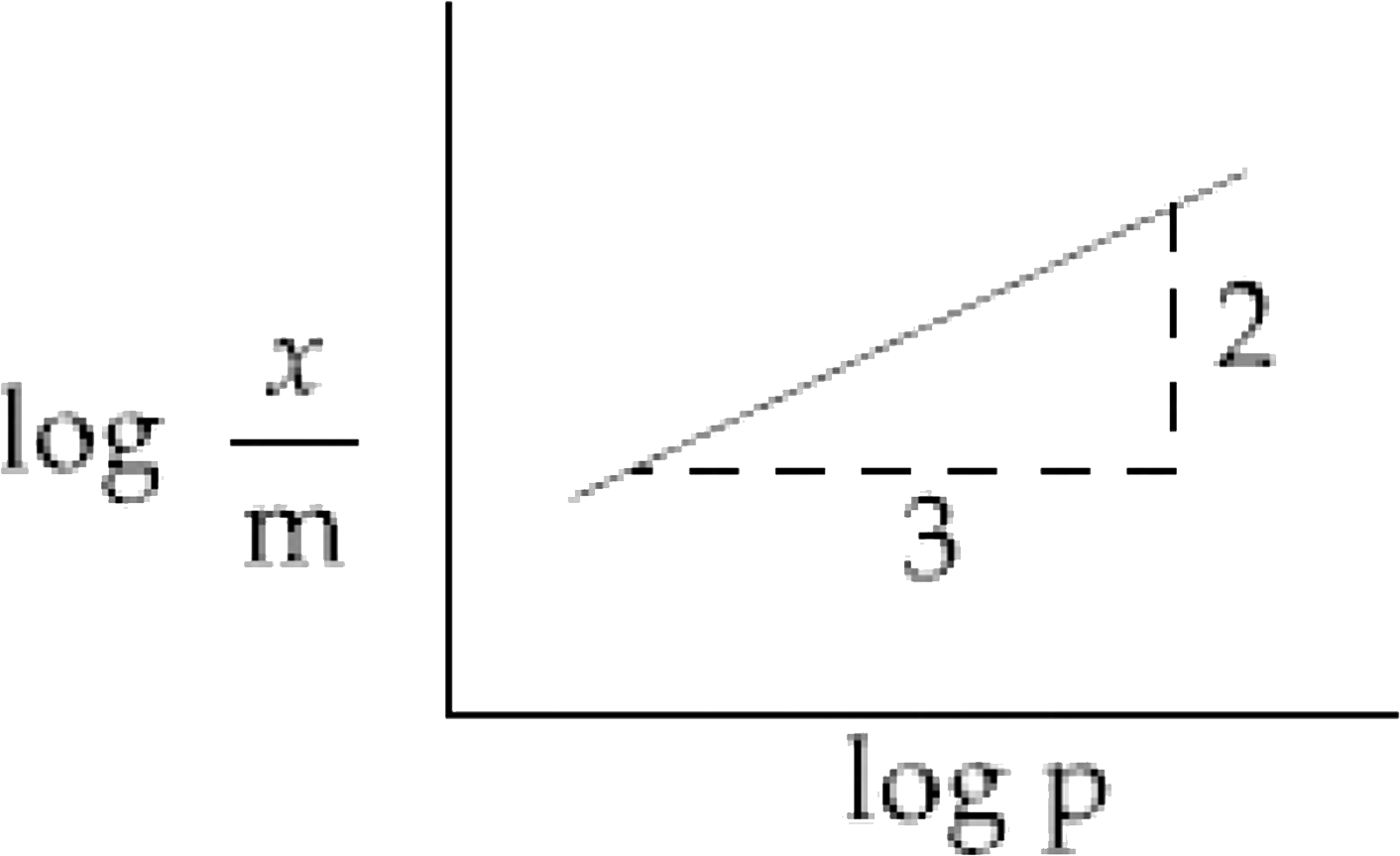
View SolutionAdsorption of gas follows Freundlich adsorption isotherm. x is the mass of the gas adsorbed on mass m of the adsorbent. The plot of logxm versus log p is shown in the given graph.
xm is proportional to:

View SolutionWhat will be the Freundlich's adsorption isotherm equation at high pressure?
View SolutionIn Freundlich adsorption isotherm, adsorption is proportional to pressure P as
View Solution

