Identify relation that is incorrect
A
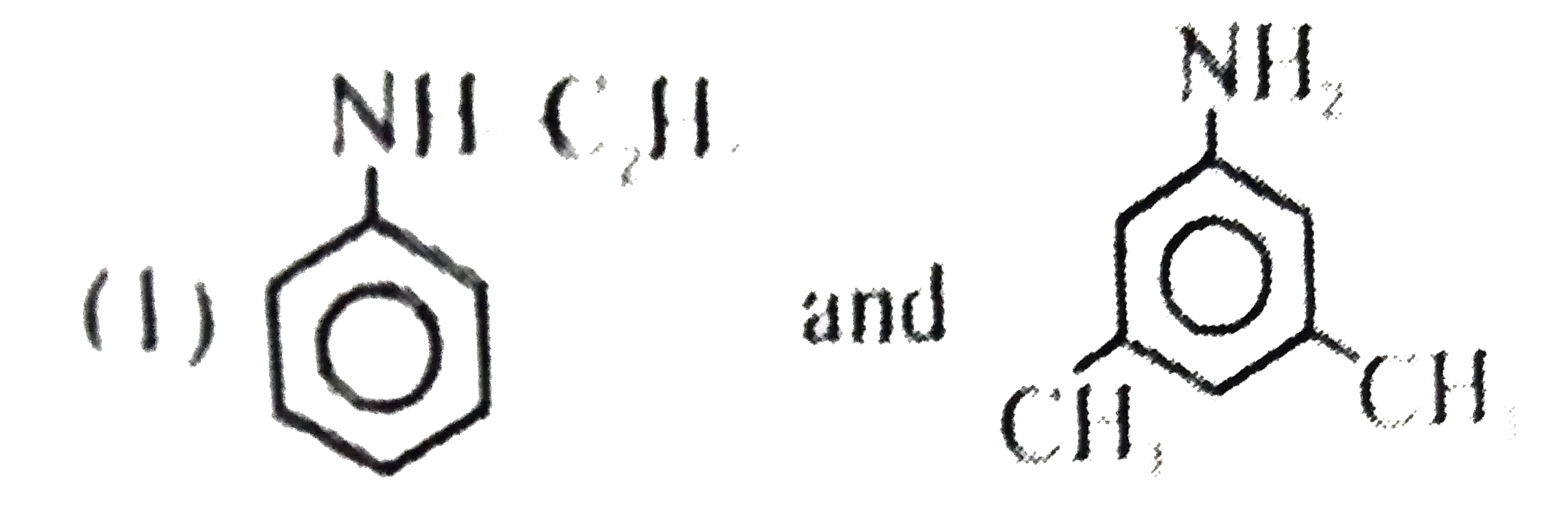
B

C
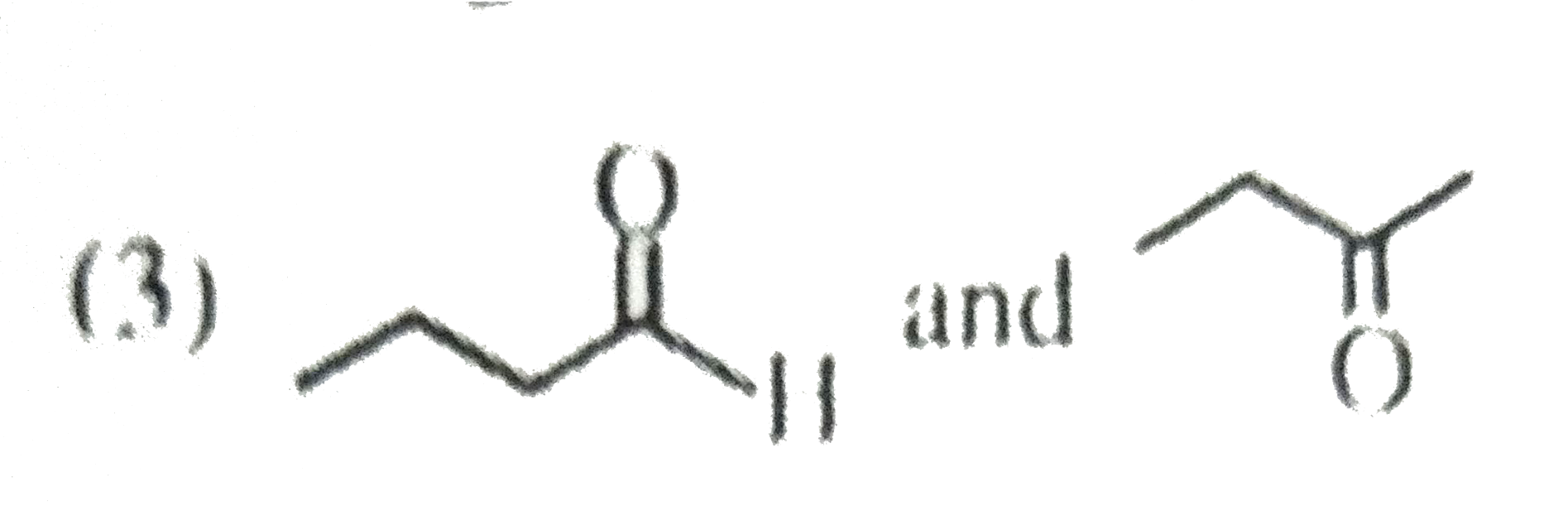
D
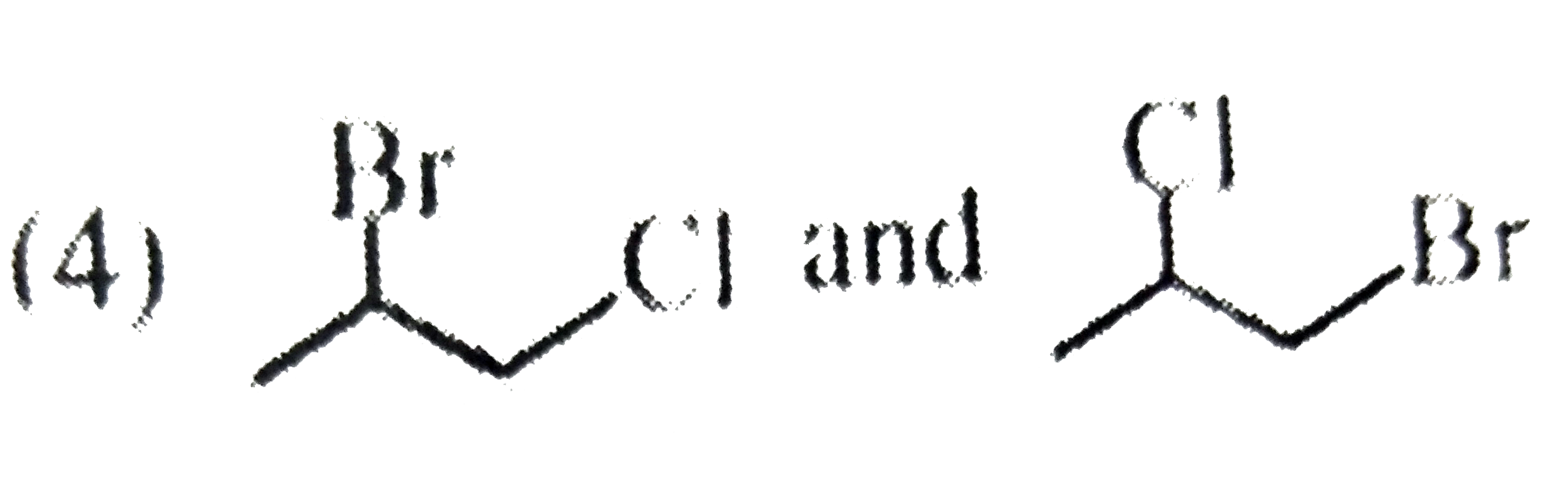
Text Solution
The correct Answer is:C
|
Similar Questions
Knowledge Check
Identify the only incorrect statement.
AFe3+ has greater electron affinity than Fe2+BBetween F and CI, formation of CI− is more exothermic than formation of F−CIE1 of CI−= electron affinity of CIDElectronegativity depends only on electron affinityIncorrect relation is
Afor isothermal reversible change W=−Pext(Vf−Vi)Bfor isothermal reversible change Q=2.303nRtlogVfViCfor isothermal reversible change W=−2.303nRTlogPfPiDfor adiabatic change ΔU=WIdentify the incorrect statement related to PCI5 from the following:
AThree equatorial P-Cl bonds make an angle of 120∘ with each otherBTwo axial P-Cl bonds make an angle of 180∘ with each otherCAxial P-Cl bonds are longer than equatorial P-Cl bondsDPCI5 molecule is non-reactive
Identify the incorrect pair
View Solutionidentify incorrect pair
View SolutionIdentify the incorrect one in relation to human reproduction
View SolutionIdentify the incorrect statement related to PCI5 from the following:
View SolutionIdentify the incorrect statement(s) in relation to C4- photosynthesis .
View Solution

