In a cylinder-piston .............
The correct Answer is:40.3
Isothermal process.
(P1+4Tr)(43πr3)=(P2+4Tr/2)(43π(r/2)3)
P2=8P(1)+24Tr
Similar Questions
2 moles of a diatomic gas are enclosed in a cylinder piston arrangment. The area of cross section and mass of the piston are 1cm2 and 1 kg respectively. A heater is supplying heat to the gas very slowly. Find heat supplied (in joule) by the heater is the piston moves through a distance of 10 cm.
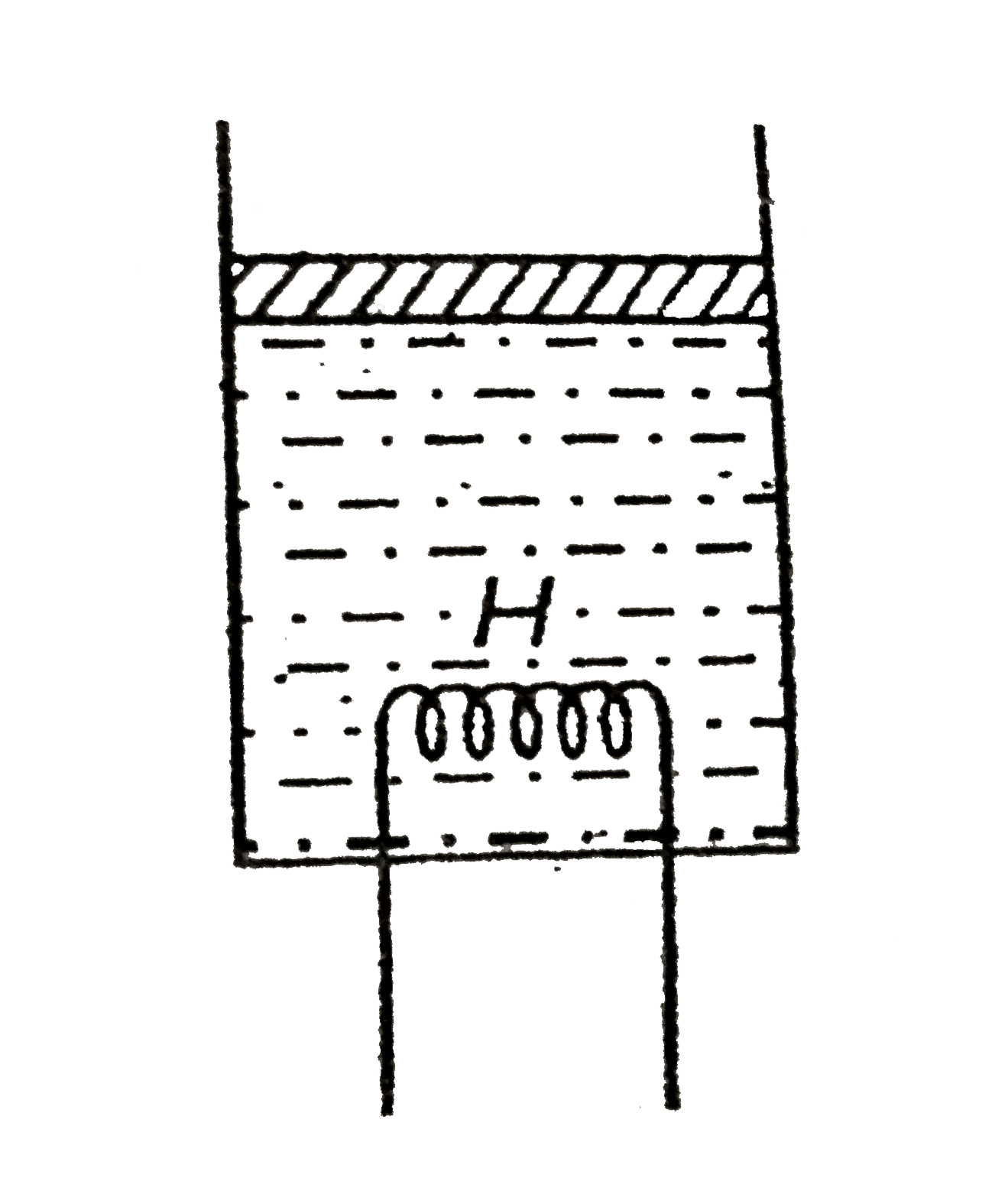 .
.
View Solution2 moles of a diatomic gas are enclosed in a cylinder piston arrangment. The area of cross section and mass of the piston are 1cm2 and 1 kg respectively. A heater is supplying heat to the gas very slowly. Find heat supplied (in joule) by the heater is the piston moves through a distance of 10 cm.
 .
.
View Solution
Knowledge Check
A cycle followed an engine (made of one mole of an ideal gas in a cylinder with a piston) is shown in figure The heat exchanged by the engine with the surroundings at constant volume is (Take CV=32R)
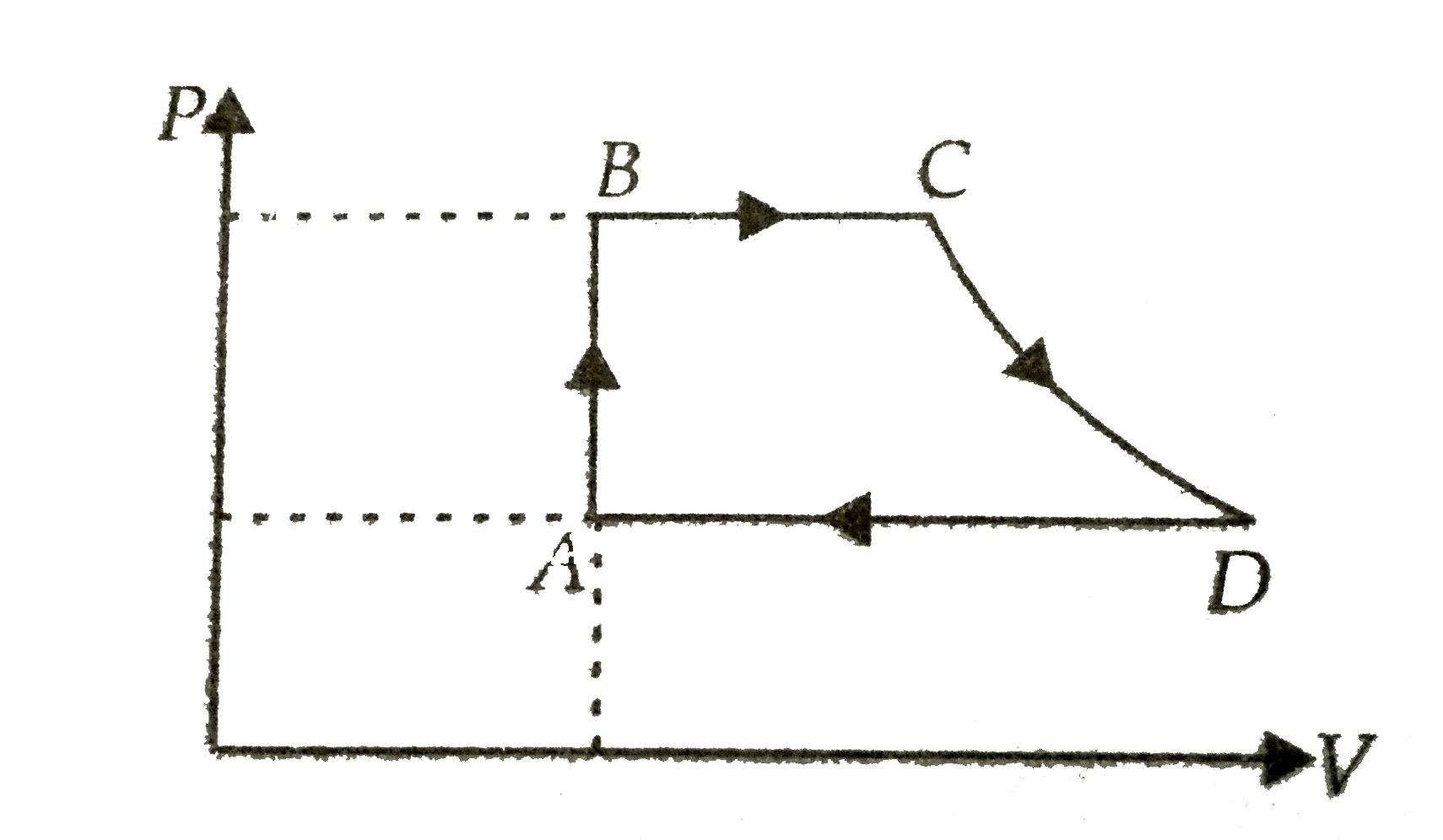 A(PB−PA)VAB12(PB−PA)VAC32(PB−PA)VAD52(PB−PA)VA
A(PB−PA)VAB12(PB−PA)VAC32(PB−PA)VAD52(PB−PA)VAThe cycle in the figure followed by an engine made of an ideal gas in ca cylinder with a piston, the heat exchanged by the engine with the surroundings for adiabatic section AB of cycle is (CV=32R)
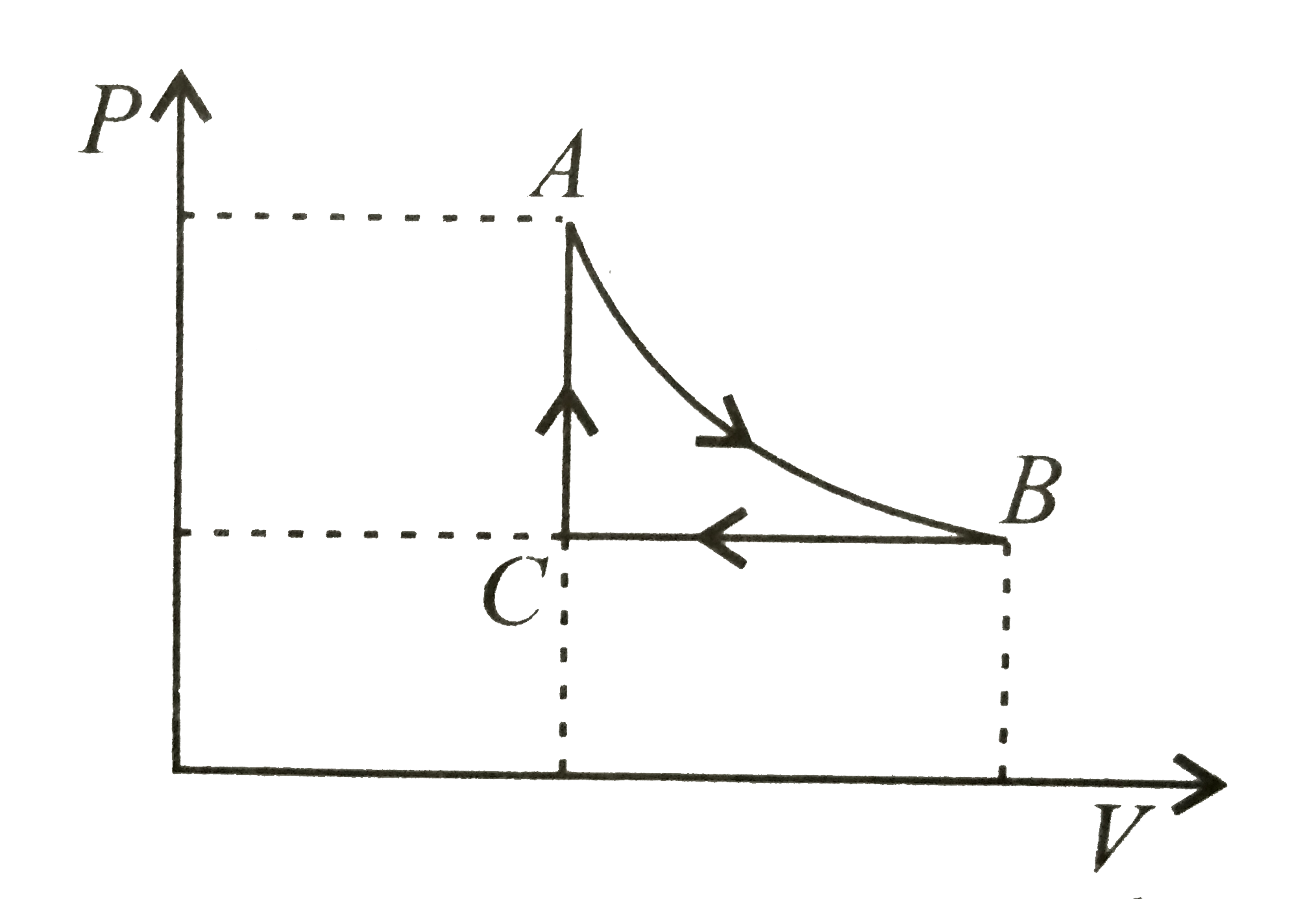 A32(PB−PA)VAB52PA(VA−VB)C12(PA−PB)(VA−VB)DZero
A32(PB−PA)VAB52PA(VA−VB)C12(PA−PB)(VA−VB)DZero
A thermodynamic system consists of a cylinder-piston attangement with ideal gas in it. It goes from the state i to the state f as shwon in the figure. The work done by gas during the process is
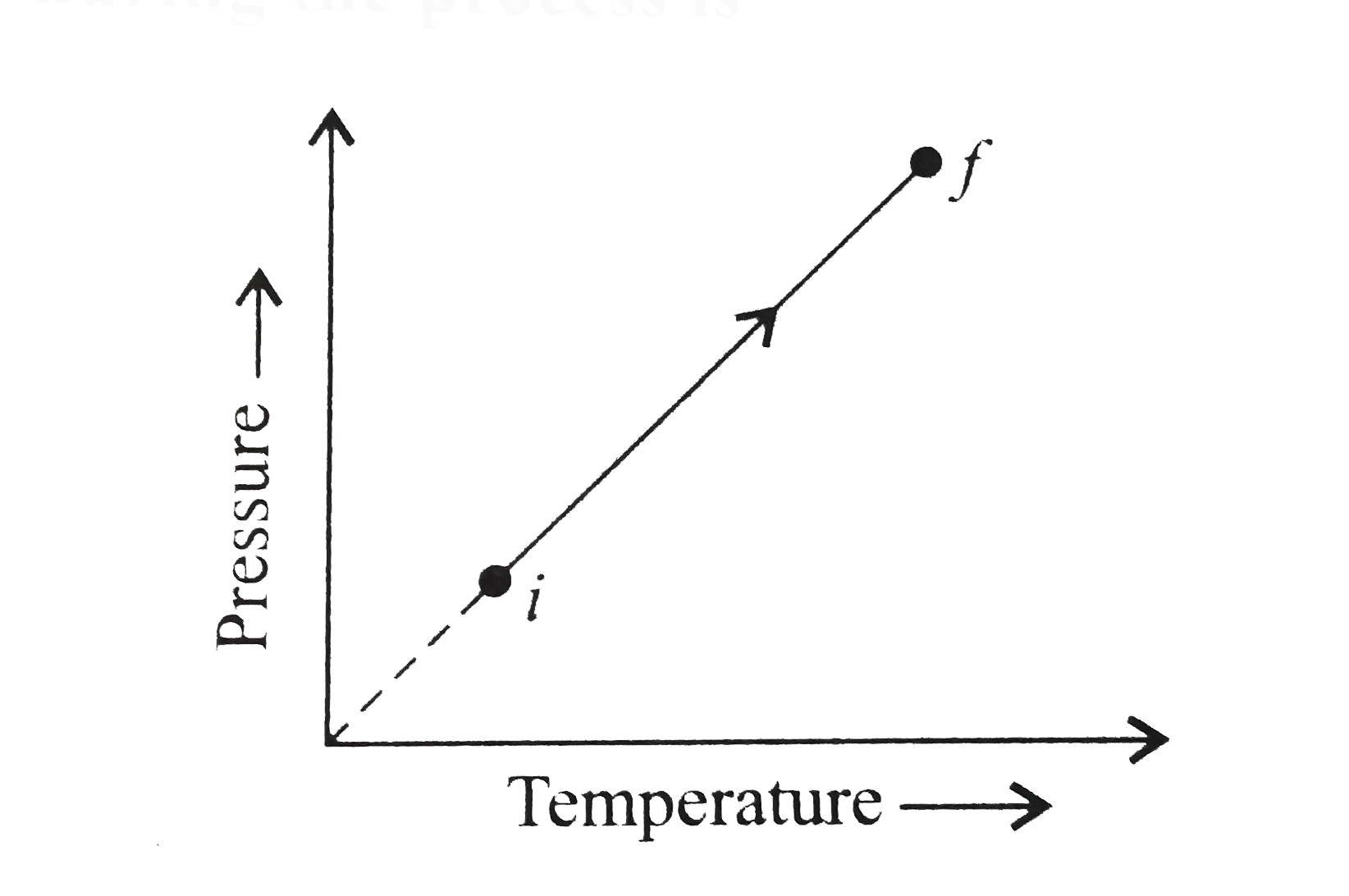
View SolutionA thermodynamic system consists of a cylinder-piston attangement with ideal gas in it. It goes from the state i to the state f as shwon in the figure. The work done by gas during the process is

View SolutionIn the figure shown below, cylinder A has pump piston, whereas B and C cylinder have lift pistons. If the maximum weight that can be placed on the pump piston is 50 kgwt, what is the maximum weight that can be lifted by piston in the cylinder B? Find the total mechanical adventage.
(Take g 10 m s−2).
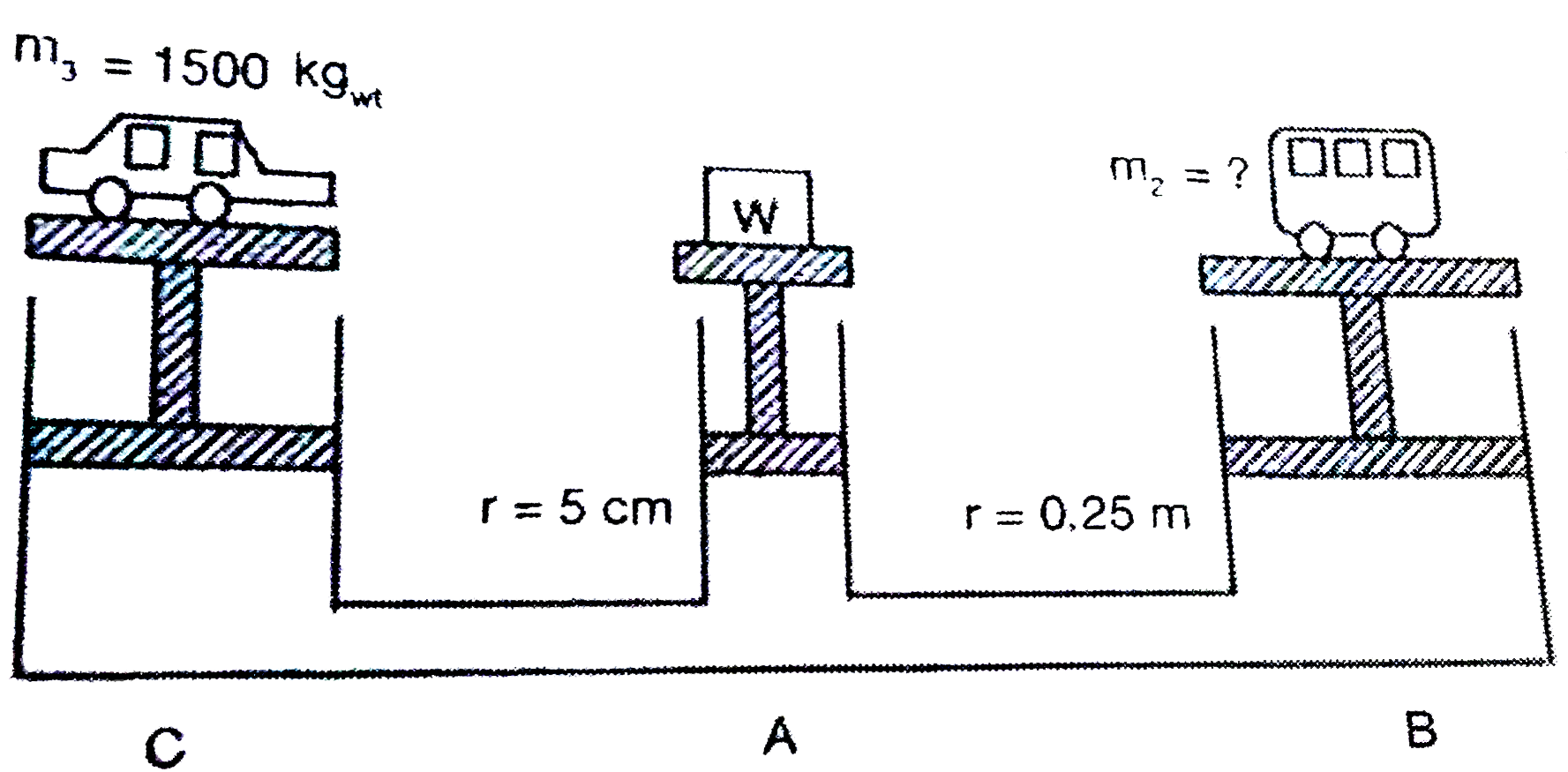
View SolutionA gas is filled in a cylinder fitted with a piston at a definite temperature and pressure. Explain on the basis of kinetic theory why on pulling the piston out, the pressure of decreases.
View SolutionA gas is filled in a cylinder fitted with a piston at a definite temperature and pressure. Explain on the basis of kinetic theory why on pulling the piston out, the pressure of decreases.
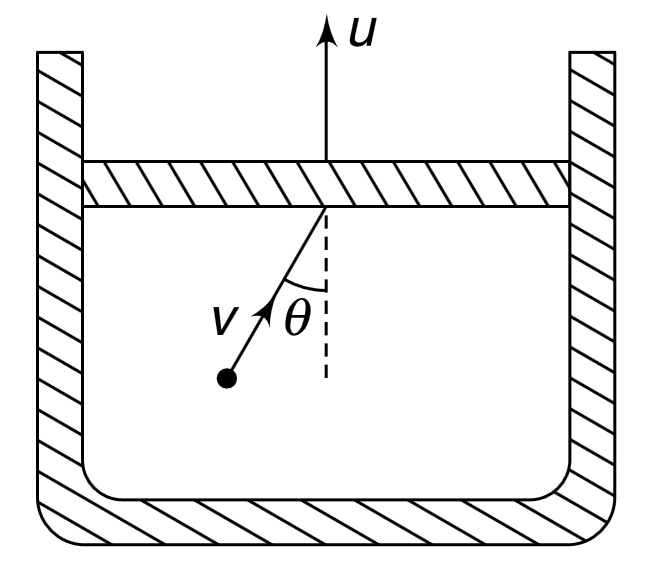
View SolutionAn ideal gas is inside a cylinder with a piston that can move freely. The walls of the cylinder and piston are non- conducting. The piston is being moved out of the cyl- inder at a constant speed u.
(a) Consider a gas molecule of mass m moving with speed v(>>u). It hits the piston elastically at an angle of incidence q. Calculate the loss in kinetic energy of the molecule.
(b) If the area of piston is A and pressure of the gas is P, calculate the rate of decreases of molecular kinetic energy of the gas sample.
(c) Ifu>> molecular velocities, at what rate will the gas lose its molecular kinetic energy
View Solution

