An aqueous ..............
The correct Answer is:1
Borax is not any redox agent, not a bleach. Its aqueous solution turns red litmus blue & it can be titrated by titrating against HCl.
Na2B4O7.10H2O+HCl→2NACl+4H3BO3+4H2O
Topper's Solved these Questions
Similar Questions
A simple pendulum consisting of a mass M attached to a string of length L is released from rest at an angle α. A pin is located at a distance l below the pivot point. When the pendulum swings down, the string hits the pin as shown in figure. The maximum angle θ which the string makes with the vertical after hitting the pin is
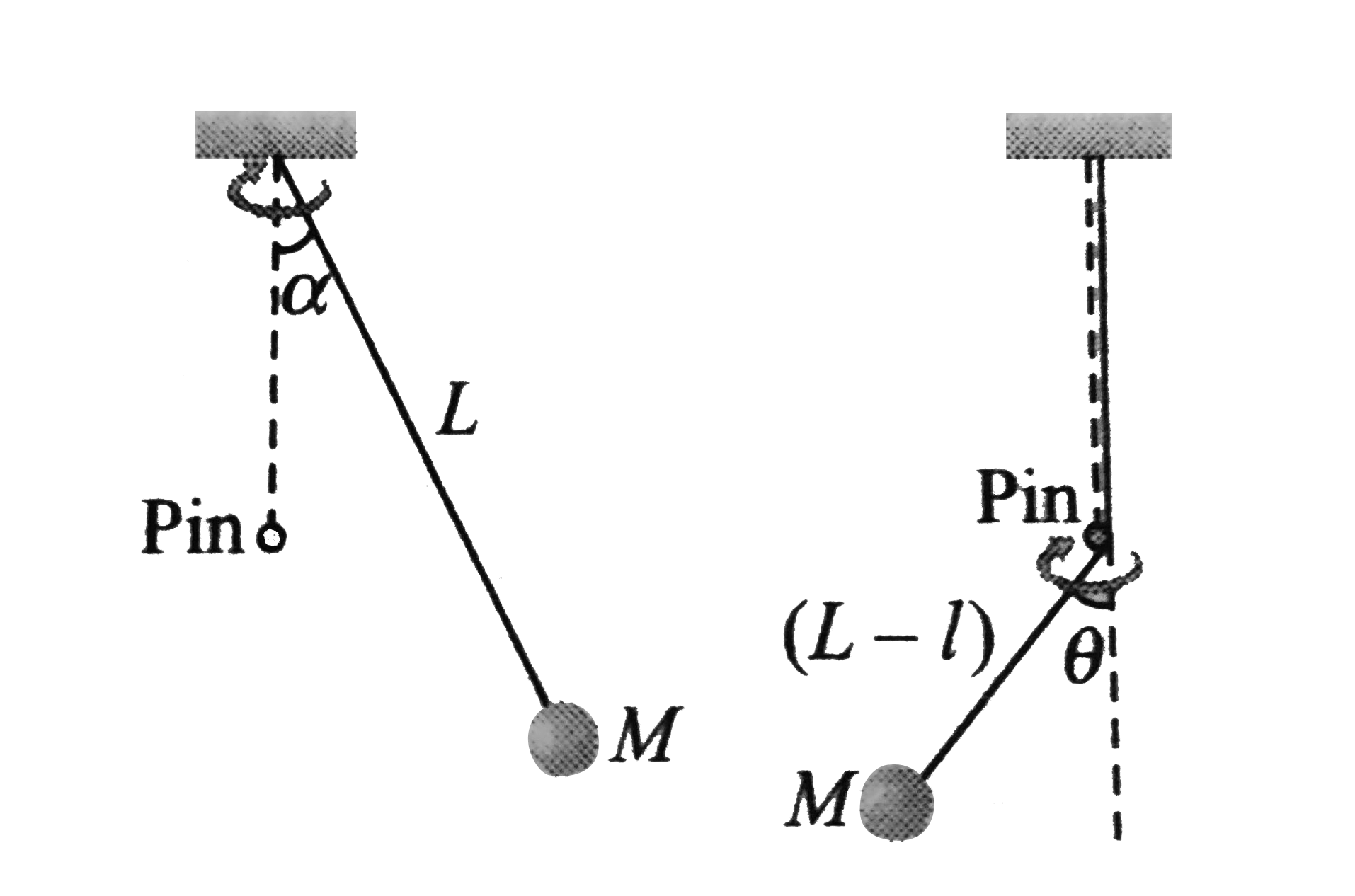
View SolutionIn a book with page numbers from 1 to 100, sme pages are torn off. The sum of the numbers on the remaining pages is 4949. How many pages are torn off ?
View SolutionThe least positive integral value of 'k' for which there exists at least one line that the tangent to the graph of the curve y=x3−kx at one point and normal to the graph at another point is
View SolutionA' is an aqueous acid , 'B' is an aqueous base. If they are diluted separately , then
View Solutionजलीय ऐलुमिना की क्रिया जलीय NaOH के साथ की जाती है। अभिक्रियाओं को समझाइए ।
View Solutionजलीय और अजलीय विलयन में अन्तर बताओं |
View Solutionजलीय ऐलुमिना की क्रिया जलीय NaOH के साथ की जाती है। अभिक्रियाओं को समझाइए ।
View SolutionWhat is aqueous and non - aqueous solution ?
View Solutionaqueous solution : Water :: Non - aqueous solution :______.
View SolutionWhat is aqueous and non-aqueous solution? Give an example.
View SolutionA' is an aqueous acid , 'B' is an aqueous base. If they are diluted separately , then
View SolutionWhat is aqueous and non - aqueous solution ?
View SolutionWhat are aqueous and non-aqueous solution? Give example.
View SolutionWhat is aqueous and non-aqueous solution? Give an example.
View SolutionA is an aqueous acid, B is an aqueous base. They are diluted separately, then
View Solution

