A green mass is formed in the charcoal cavity test when a colourless salt (X) is fused with cobalt nitrate. X contains
Al3+
Cu2+
Ba2+
Zn2+
Green colour formed is due to ZnO.Co O
Similar Questions
A green mass is formed in the charcoal cavity test when a colourless salt (X) is fused with cobalt nitrate. (X) may contain
View SolutionAl salts on heating with Na2CO3 in charcoal cavity gives a white infusible residue. On moistening the same with a drop of cobalt nitrate, it changes to ____due to the formation of cobalt meta-aluminate (Thenard's blue)
View Solution
Knowledge Check
A green mass is formed in the charcoul cavity test when a colourless salt (X) is fussed with chbalt mitrate (X) may contain
AAluminiumBcopperCBariumDZineAl salts on heating with Na2CO3 in charcoal cavity gives a white infusible residue. On moistening the same with a drop of cobalt nitrate, it changes to ____due to the formation of cobalt meta-aluminate (Thenard's blue)
ABrownBYellowCBlueDLemonA blue coloured residue obtained in cobalt nitrate charcoal cavity test is due to
AZn2+BMg2+CSn2+DAl3+
X' is a colourless salt giving following reactions :
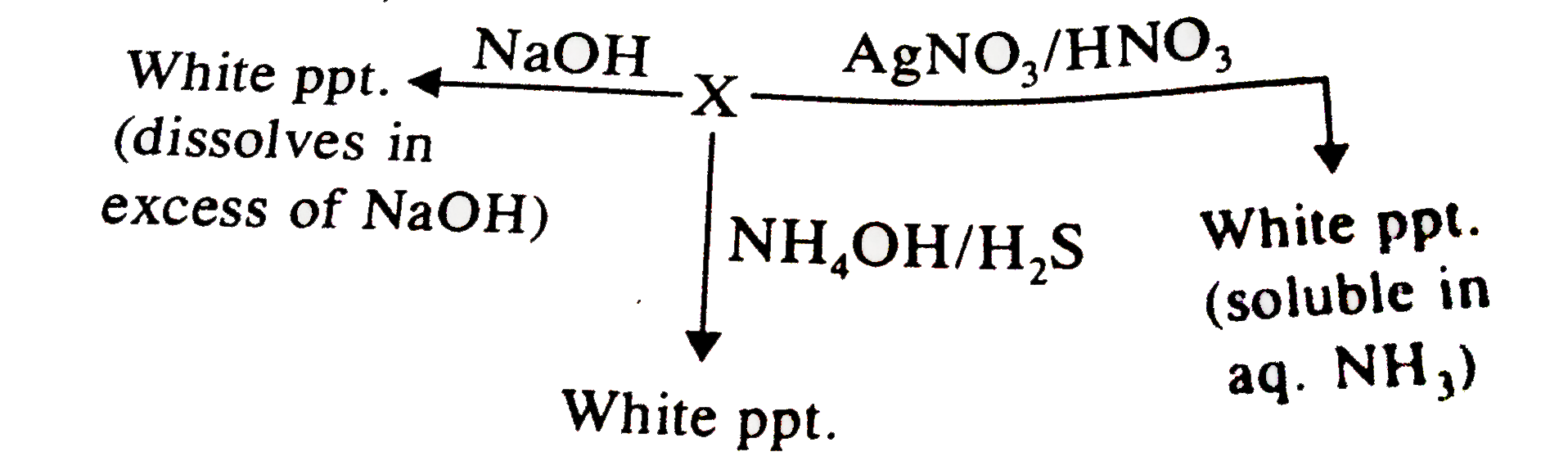
'X' can be
View SolutionA white solid forms Rinmann's greenn in the charcoal cavity test in an oxidising flame. On treatment with dilute H2SO4, this solid produces a gas that turns an acidified dichromate paper green ad lead acetate paper black. The white solid is:
View SolutionX' is a colourless salt giving following reactions :
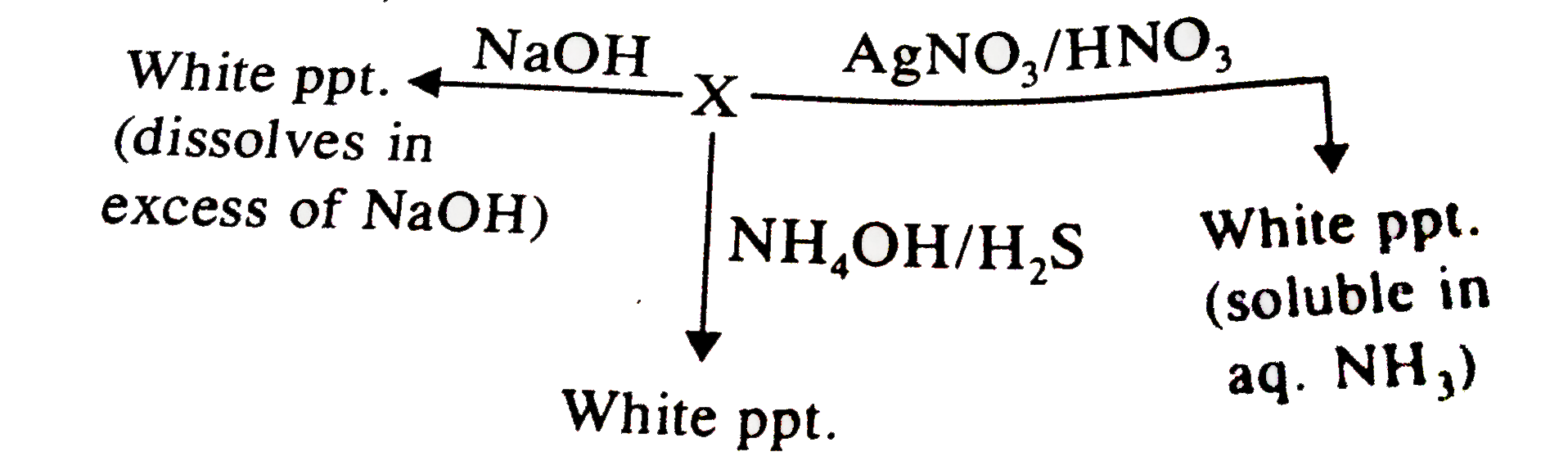
'X' can be
View SolutionA white solid forms Rinmann's greenn in the charcoal cavity test in an oxidising flame. On treatment with dilute H2SO4, this solid produces a gas that turns an acidified dichromate paper green ad lead acetate paper black. The white solid is:
View SolutionWhich of the following salt gives green colour mass in cobalt nitrate charcoal cavity test?
View Solution

