What is the role of metal or reagents written on arrows in the given chemical reactions ?
(a) 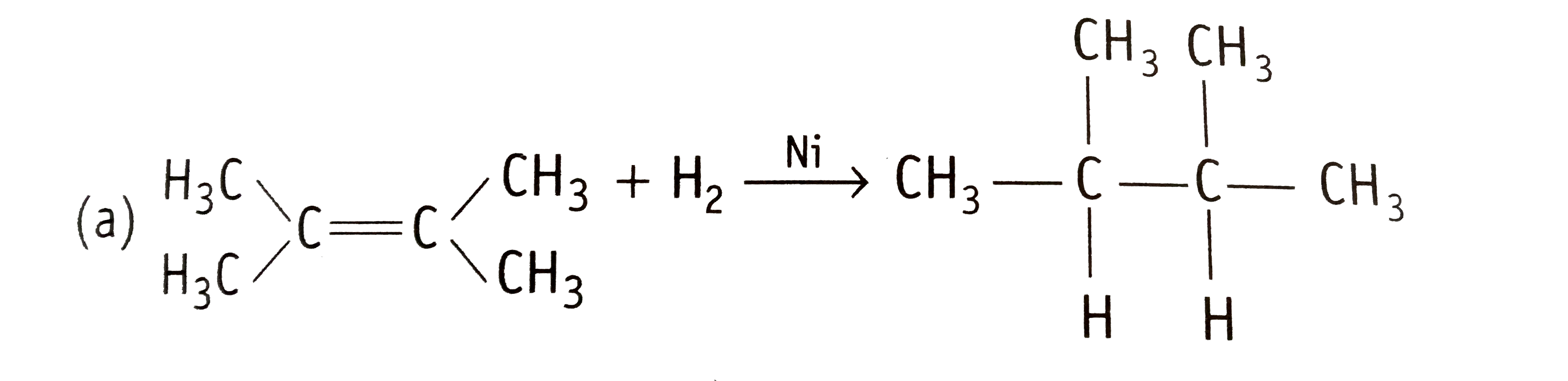
(b) CH3COOH+CH3CH2OHConc.H2SO4−−−−−−−→CH3COOC2H5+H2O
(c ) CH3CH2OHAlk.KMnO4−−−−−−−→HeatCH3COOH
(a) Nickel (Ni) acts as a hydrogenation catalyst for the reaction.
(b) Conc. H2SO4 removes a molecule of H2O from the reaction mixture and acts as a dehydrating agent.
(c) Alkaline KMnO4 acts as an oxidising agent and oxidises alcohol to ethanoic acid.
Similar Questions
Match the reactions given in Column (A) with the names given in Column (B).
Column AColumn B(a)CH3OH+CH3COOHH+−−→CH3COOCH3+H2O(i)Addition reaction(b)CH2=CH2+H2Ni−−→CH3−CH3(ii)Substitution reaction(c)CH4+Cl2Sunlight−−−−→CH3Cl+HCl(iii)Neutralisation reaction(d)CH3COOH+NaOH→CH3COONa+H2O(iv)Esterification reaction
View SolutionCH3COOH+C2H5OHconc.H2SO4−−−−−−→heatCH3COOC2H5+H2O
The above reaction is known as
View SolutionCH3−CH2−OHAlkalineKMnO4+Heat−−−−−−−−−−−−−→CH3−COOH
In the above given reaction, alkaline KMnO4 acts as
View SolutionWhat is the role of metal or reagents written on arrows in the given chemical reactions ?
(a)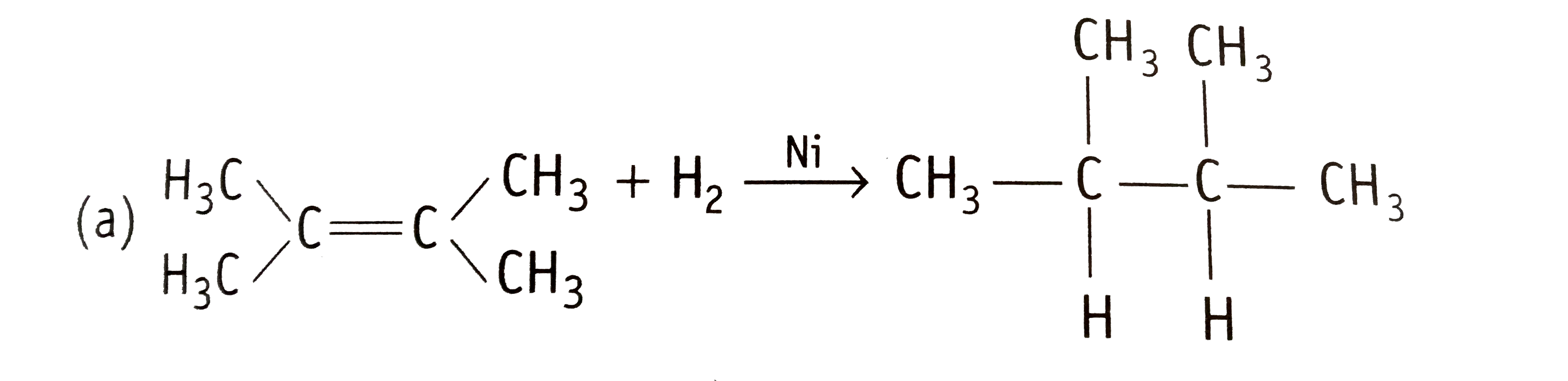
(b) CH3COOH+CH3CH2OHConc.H2SO4−−−−−−−→CH3COOC2H5+H2O
(c ) CH3CH2OHAlk.KMnO4−−−−−−−→HeatCH3COOH
View SolutionWrite mechanism of the following reaction:
CH3CH2OHHBr−−→CH3CH2Br+H2O
View SolutionCH_(3)CH_(2)OH overset("Alkaline "KMnO_(4)+"Heat")toCH_(3)COOH In the above given reaction, alkaline KMnO_(4) acts as …
View SolutionState the role of reagents shown on arrows in the following chemical reactions. CH_(3)COOH+CH_(3)CH_(2)OHoverset("Conc."H_(2)SO_(4))rarr CH_(3)COOC_(2)H_(5)+H_(2)O
View SolutionState the role of reagents shown on arrows in the following chemical reactions. CH_(3)CH_(2)OH overset(KMnO_(4))rarr CH_(3)COOH
View Solution

