Assertion : If U.V radiations and infrared radiations fall on a metal plate, stopping potential would be grater for infrared radiations.
Reason : Maximum kinetic energy of photoelectrons is inversely proportional to the frequency of incident radiation.
If both assertion and reason are correct and reason is a correct explanation of the assertion.
If both assertion and reason are correct but reason is not the correct explanation of assertion.
If assertion is correct but reason is incorrect
If both assertion and reason are incorrect.
The correct Answer is:D
To solve the given question, we need to analyze both the assertion and the reason provided.
1. Understanding the Assertion:
- The assertion states that if UV and infrared radiations fall on a metal plate, the stopping potential would be greater for infrared radiations.
- Stopping potential (V) is related to the maximum kinetic energy (K.E.) of the emitted photoelectrons, which is given by the equation:
K.E.=hν−hν0
where h is Planck's constant, ν is the frequency of the incident radiation, and ν0 is the threshold frequency.
2. Analyzing the Energies of UV and Infrared:
- UV radiation has a higher frequency than infrared radiation. Since energy is directly proportional to frequency (E=hν), UV radiation has more energy than infrared radiation.
- Therefore, when UV radiation is incident on the metal plate, it will produce photoelectrons with greater kinetic energy compared to infrared radiation.
3. Conclusion on the Assertion:
- Since the stopping potential is greater for radiation with higher energy (higher frequency), the assertion that stopping potential would be greater for infrared radiations is incorrect.
4. Understanding the Reason:
- The reason states that the maximum kinetic energy of photoelectrons is inversely proportional to the frequency of incident radiation.
- This statement is incorrect. The correct relationship is that the maximum kinetic energy of photoelectrons is directly proportional to the frequency of the incident radiation, as shown in the equation above.
5. Conclusion on the Reason:
- Since the reason is also incorrect, we conclude that both the assertion and the reason are false.
Final Answer:
Both the assertion and the reason are incorrect.
- The assertion states that if UV and infrared radiations fall on a metal plate, the stopping potential would be greater for infrared radiations.
- Stopping potential (V) is related to the maximum kinetic energy (K.E.) of the emitted photoelectrons, which is given by the equation:
K.E.=hν−hν0
where h is Planck's constant, ν is the frequency of the incident radiation, and ν0 is the threshold frequency.
- UV radiation has a higher frequency than infrared radiation. Since energy is directly proportional to frequency (E=hν), UV radiation has more energy than infrared radiation.
- Therefore, when UV radiation is incident on the metal plate, it will produce photoelectrons with greater kinetic energy compared to infrared radiation.
- Since the stopping potential is greater for radiation with higher energy (higher frequency), the assertion that stopping potential would be greater for infrared radiations is incorrect.
- The reason states that the maximum kinetic energy of photoelectrons is inversely proportional to the frequency of incident radiation.
- This statement is incorrect. The correct relationship is that the maximum kinetic energy of photoelectrons is directly proportional to the frequency of the incident radiation, as shown in the equation above.
- Since the reason is also incorrect, we conclude that both the assertion and the reason are false.
Both the assertion and the reason are incorrect.
Similar Questions
Knowledge Check
Maximum kinetic energy (Ek) of a photoelectron varies with the frequency (v) of the incident radiation as
A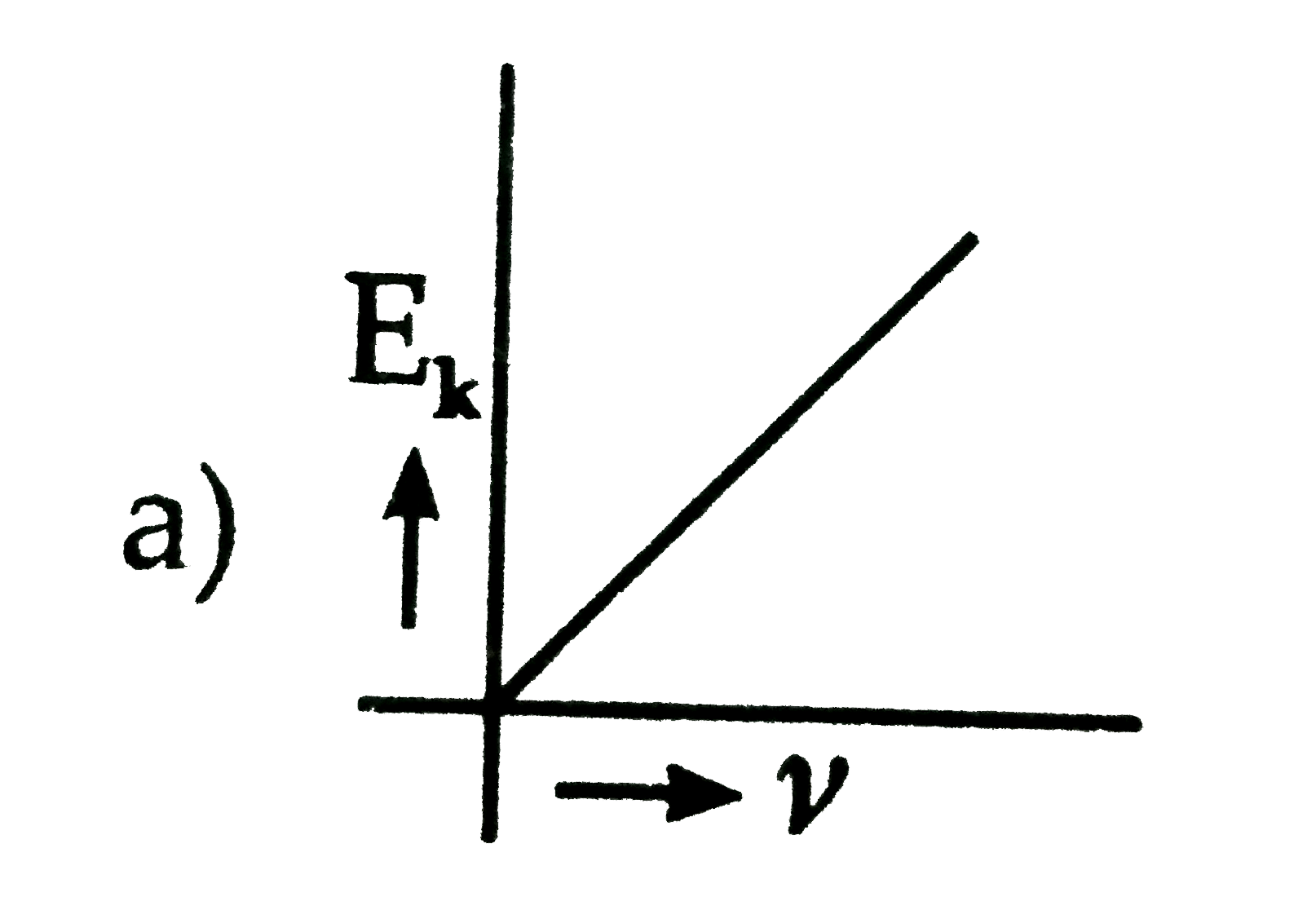 B
B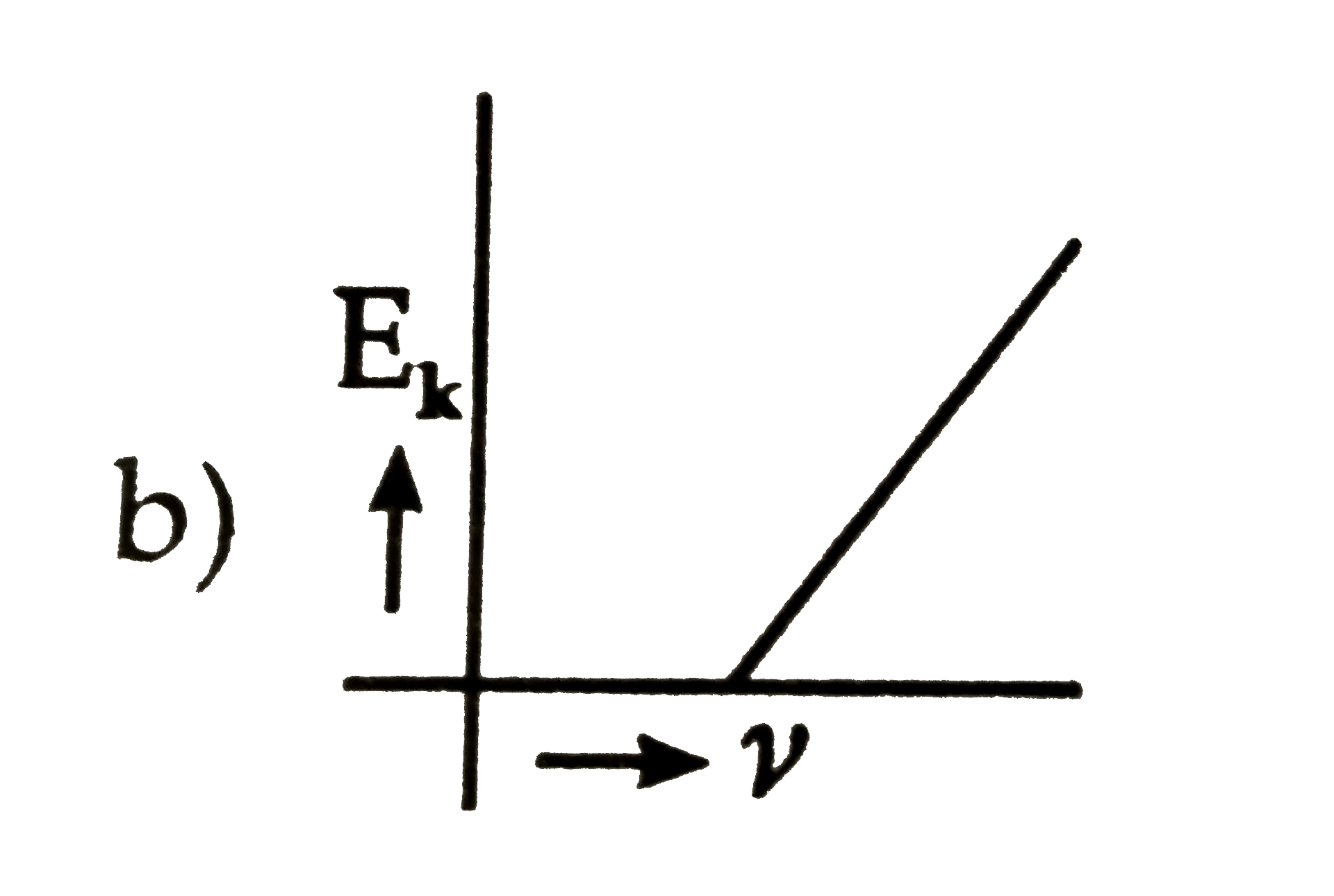 C
C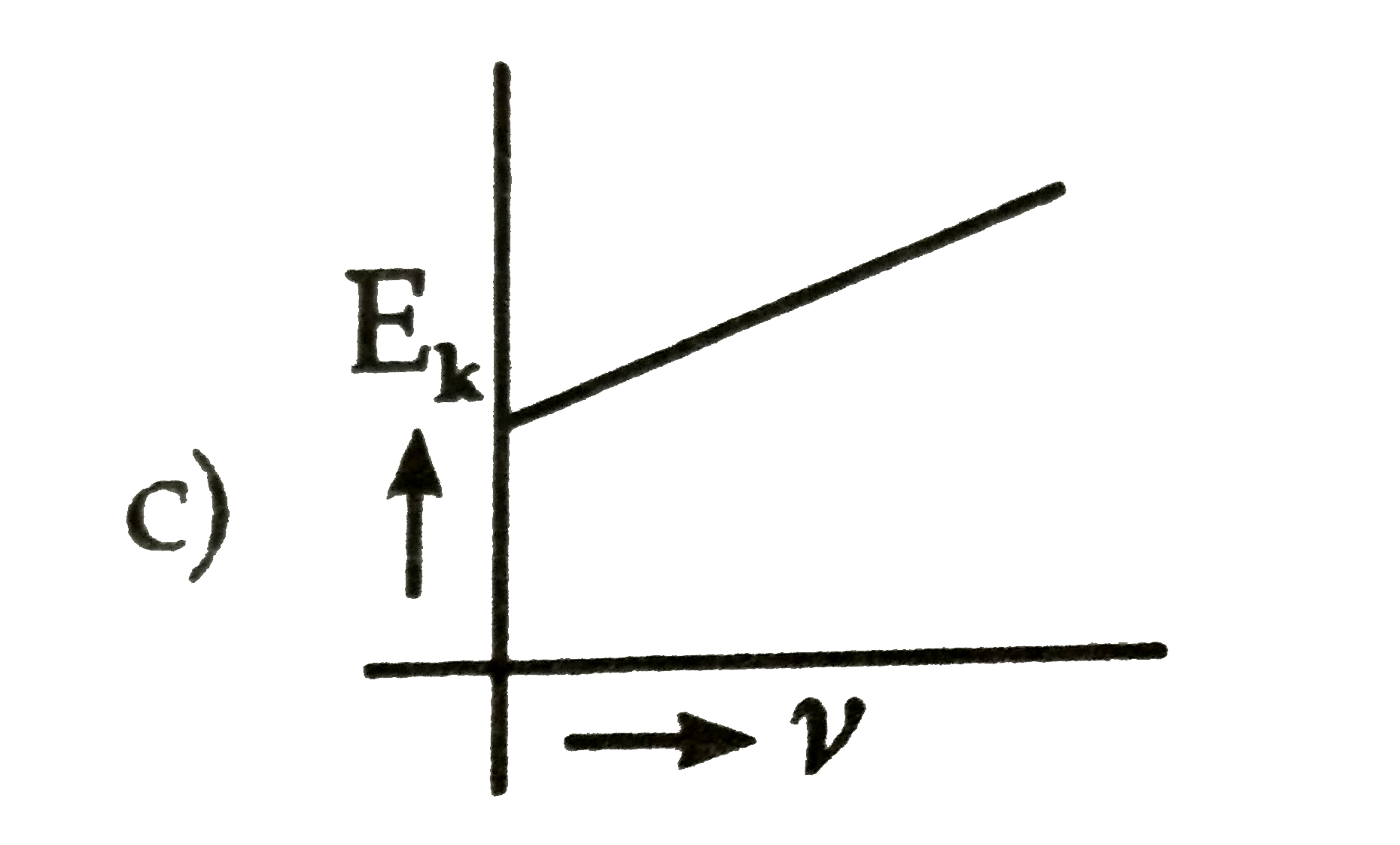 D
D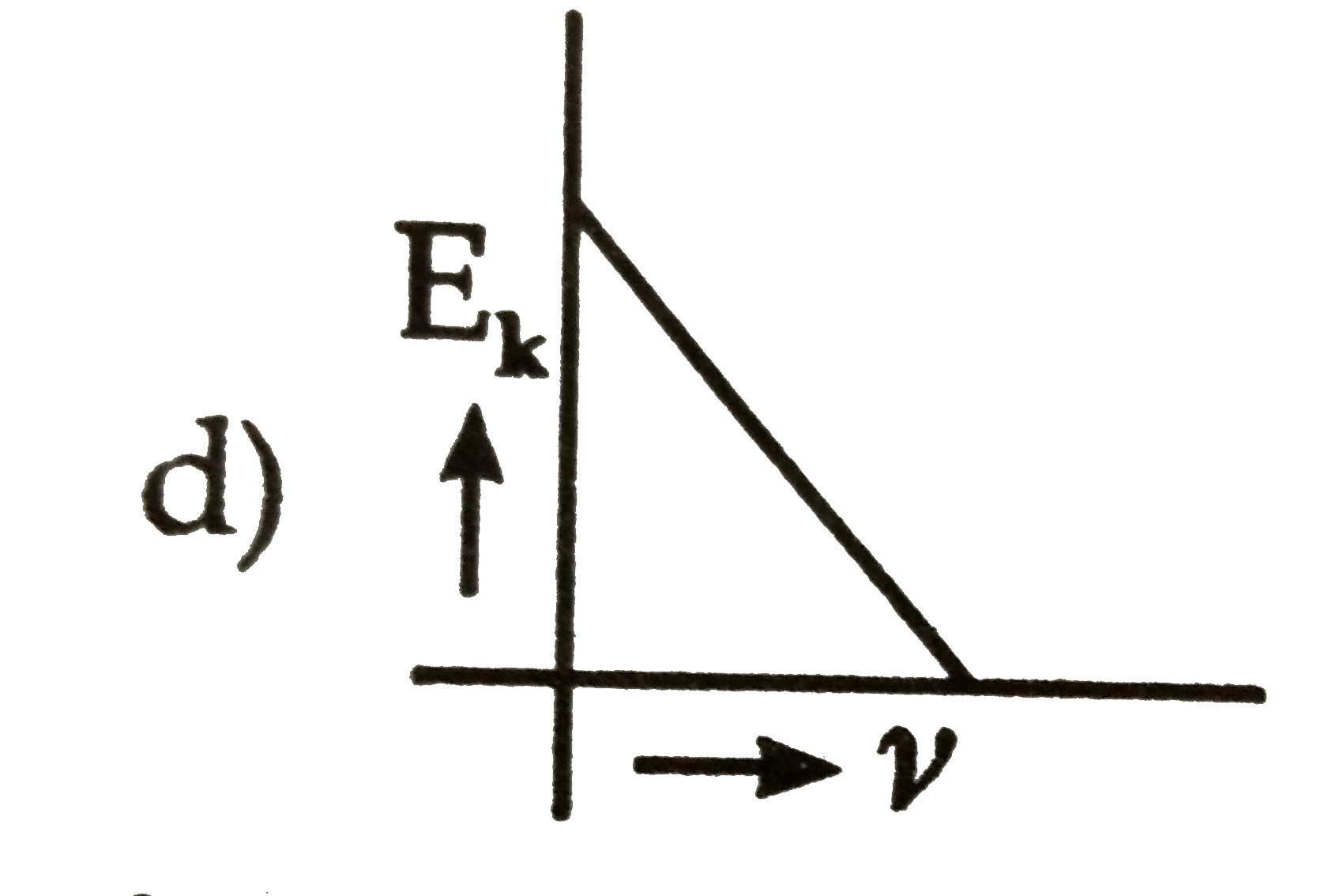
The maximum kinetic energy of photoelectrons is directly proportional to . . . Of the incident radiation. The missing word can be:
AintenstiyBwavelengthCwave numberDfrequency.According to Einstein's photoelectric equation, the graph between the kinetic energy of photoelectrons ejected and the frequency of incident radiation is
A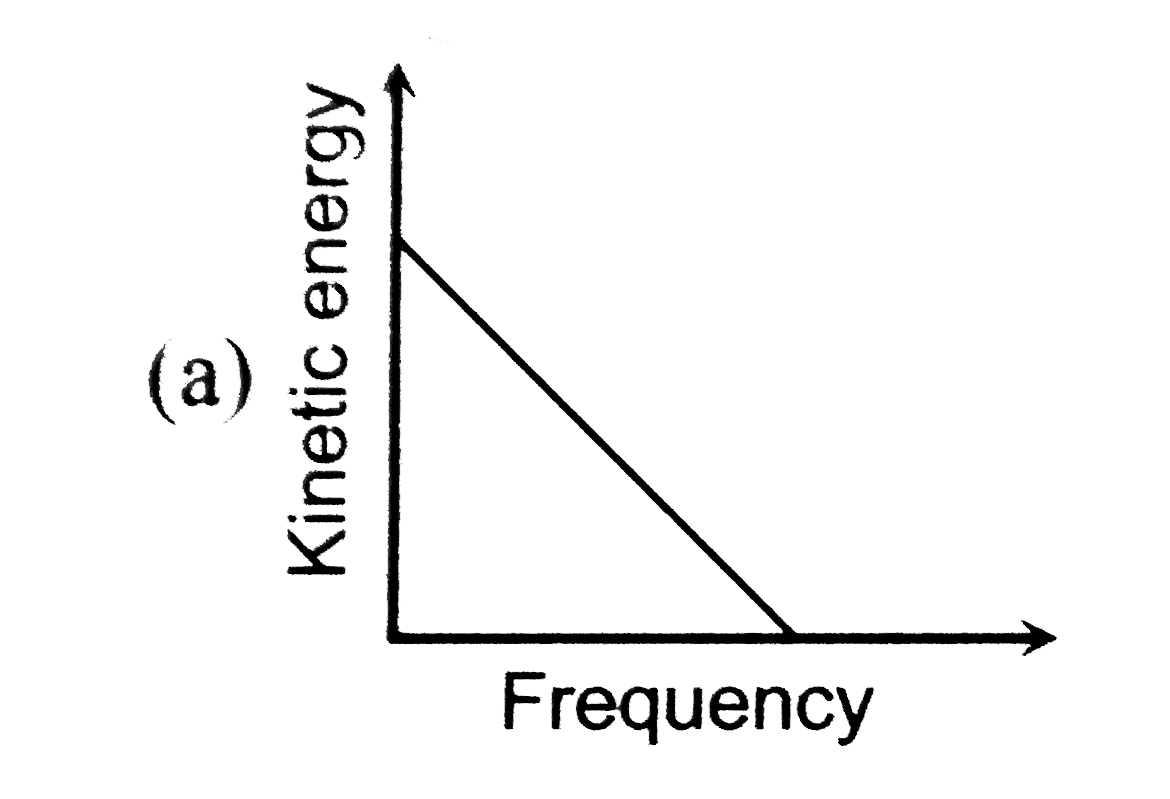 B
B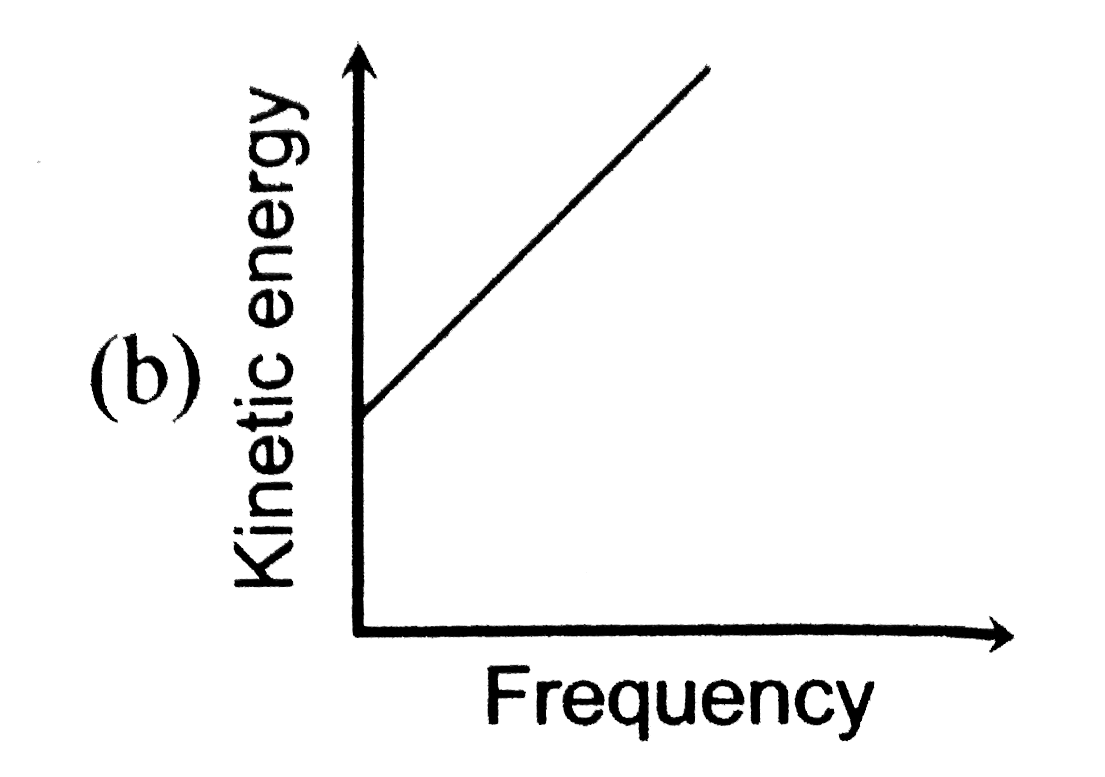 C
C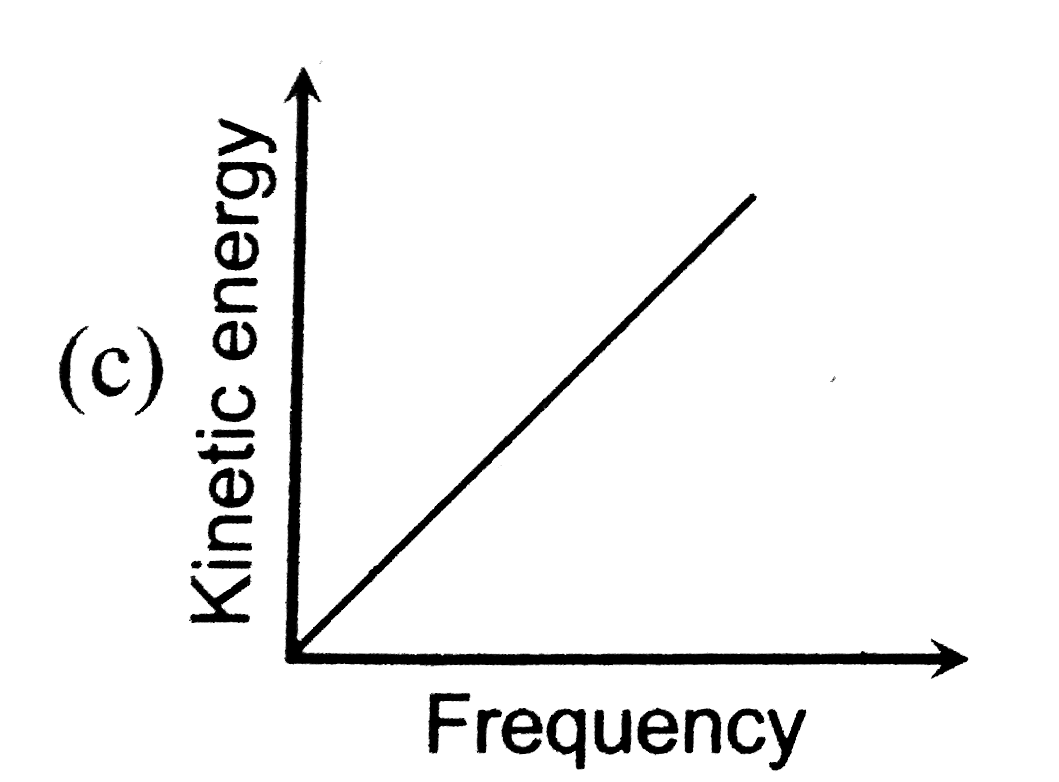 D
D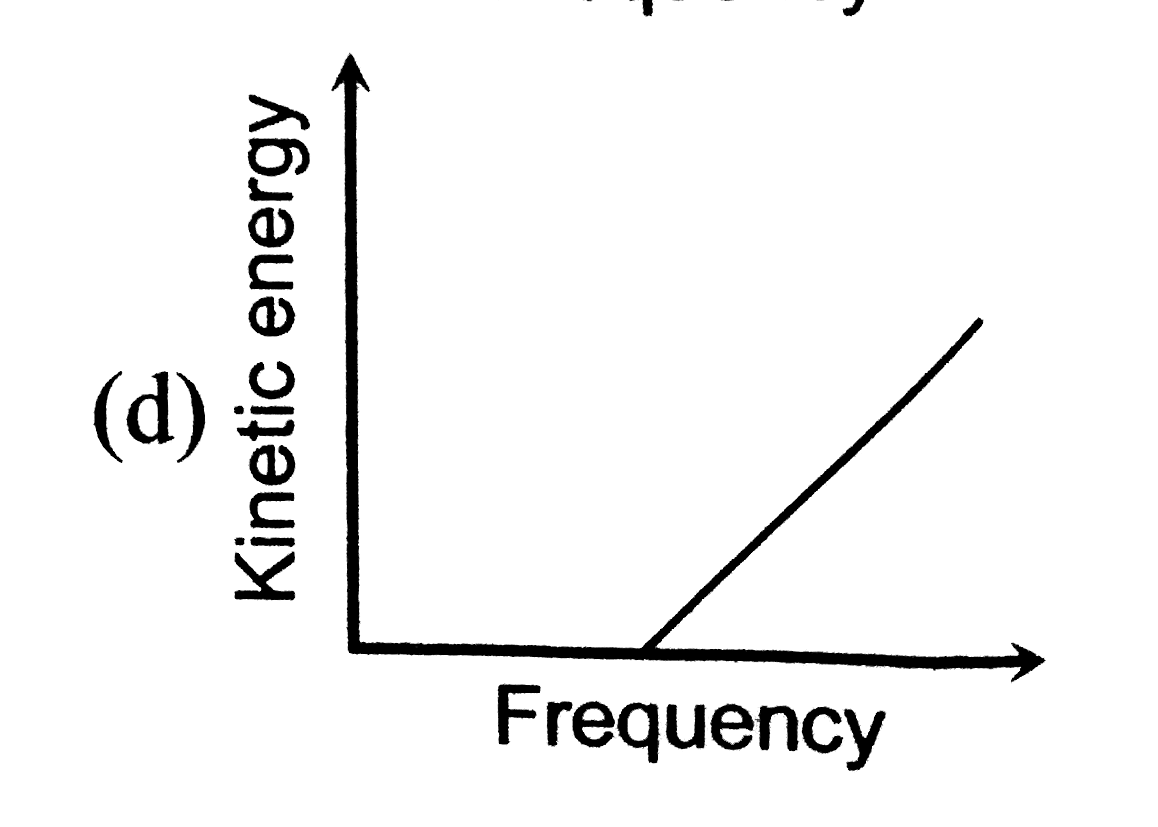
Does the kinetic energy of photoelectrons depend on the intensity of incident radiations?
View SolutionThe kinetic energy of photoelectrons emitted by a photosensitive surface depends on the internsity of the incident radiation
View SolutionAccording to Einstein's photoelectric equation, the graph between kinetic energy of photoelectrons ejected and the frequency of the incident radiation is :
View SolutionKinetic energy of photo electrons is independent on........of incident radiation.
View SolutionAssertion : Photoelectric current depends on the intensity of incident light.
Reason : Number of photoelectrons emitted per second is directly proportional to intensity of incident radiation.
View Solution
