A gyromagnetic ratio of the electron revolving in a circular orbit of hydrogen atom is 8.8×1010Ckg−1. What is the mass of the electron? Given charge of the electron =1.6×10−19C.Solution in Kannada
1×10−19c
0.1×10−29 kg
1.1×10−29kg
1/11×10−29kg
The correct Answer is:D
We know that
Magnetic moment M=iA
here i=ev2πr
M=evr2
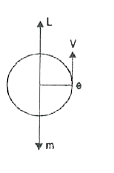
and angular momentum
divinding eq (ii) eq (ii) we get
ML=e2m
where M/L =gyromagnetic ratio
Given
M/L=8.8×1010c/kg
e=1.6×10−19C
=888×10−29
=111×10−29kg
Similar Questions
At what speed should the electron revolve around the nucleus of a hydrogen atom in order that it may not be pulled into the nucleus by electrostatic attraction ? Take the radius of orbit of an electron as 0.5Å , the mass of the electron as 9.1xx10^(-31) kg and charge as 1.6xx10^(-19)C .
View SolutionThe radius of the first orbit of electron in hydrogen atom (e=1.6xx10^(-19)" coulomb,
View SolutionA gyromagnetic ratio of the electron revolving in a circular orbit of hydrogen atom is 8.8xx10^(10)C kg^(-1) . What is the mass of the electron? Given charge of the electron =1.6xx10^(-19)C .
View SolutionIn the hydrogen atom, an electron revolve around a proton in a circular orbit of radiu 0.53 A. The radial acceleration and the angular velocity of the electron are......and…………. (m_(e) = 9.1 xx 10^(-31) kg, e = 1.6 xx 10^(-19) C)
View SolutionCalculate the gyromagnetic ratio of electron (Givene=1.6×10T−19C,me=9.1×10−31kg)
View SolutionGiven the electronic charge, e=1.6×10−19C,e≤ctronicmass,m = 9.1 xx 10^(-19) kg, h = 6.6 xx 10^(-34) J-sandc = 3 xx 10^8 m//s
The radius of the first orbit of electron in hydrogen atom is
View SolutionAt what speed should the electron revolve around the nucleus of a hydrogen atom in order that it may not be pulled into the nucleus by electrostatic attraction ? Take the radius of orbit of an electron as 0.5Å , the mass of the electron as 9.1xx10^(-31) kg and charge as 1.6xx10^(-19)C .
View Solution
