Which of the following will not form aldol when treated with dilute NaOH.
(A) \(CH_{3}CHO\)
(B) \((CH_{3})_{3}CCHO\)
(C) \(PhCH_{2}\)CHO
Step by step video & image solution for Which of the following will not form aldol when treated with dilute NaOH. (A) \(CH_{3}CHO\) (B) \((CH_{3})_{3}CCHO\)<Br>(C) \(PhCH_{2}\)CHO by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
Knowledge Check
Which of the following is not an isomer of CH3−CH2CHO
ACH3−CH2−O∣∣C−CH3B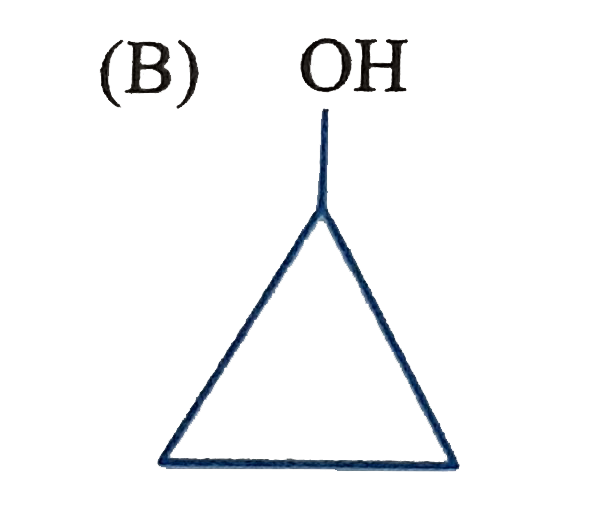 C
C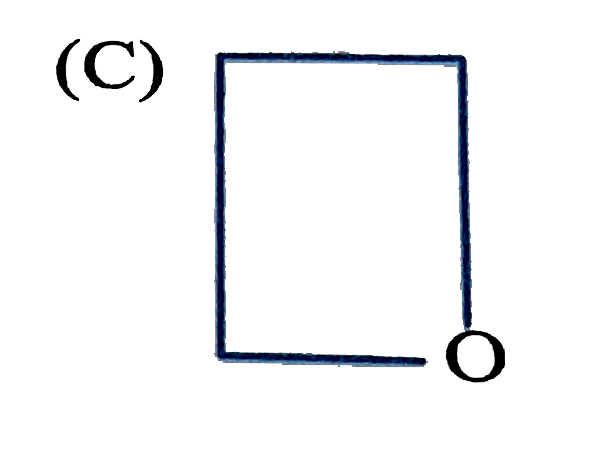 DCH2=CH−CH2−OH
DCH2=CH−CH2−OHWhich of the following undergoes cannizaro reaction ?
A)HCHO B) C6H5CHO
C) Cl3C−CHO D) (CH3)3C−CHOAOnly A & BBOnly B & CCOnly C & DDOnly A,B & DWhich of the following are examples of aldol condensation ?
1.2CH3CHOdil.NaOH−−−−−→CH3CHOHCH2CHO . 2CH3COCH3dil.NaOH−−−−−→CH3COCH(CH3)CH2COCH3
3. 2HCHOdil.NaOH−−−−−→CH3OH+HCOOH
4. C6H5CHO+HCHOdil.NaOH−−−−−→C6H5CH2OHA2,3B1 , 3C1 , 2D1,2,3
Which of the following compound does not contain pie bond.
A) CH3CONH2
B) CH3CH2OH
C) CH3CHO
D) CH3COOH
View SolutionDeduce the structures of the compounds which yeild the following products on reductive ozonolysis.
a) CH3CHO and HCHO
b) (CH3)2CO and HCHO
c) (CH3)2CO and CH3CHO
d) (CH3)2CO only
e) CH3CH2−C∣CH3=O (two moles)
f) HCHO and O=CH−CH∣CH3(CH3)2
g) CH3CH2C∣C2H5=O and OHC−CHO and OHC(CH2)CH3
h) OHC−CH2CH2CH2CHO only
i) OHC−CH2−CHO (two moles)
j)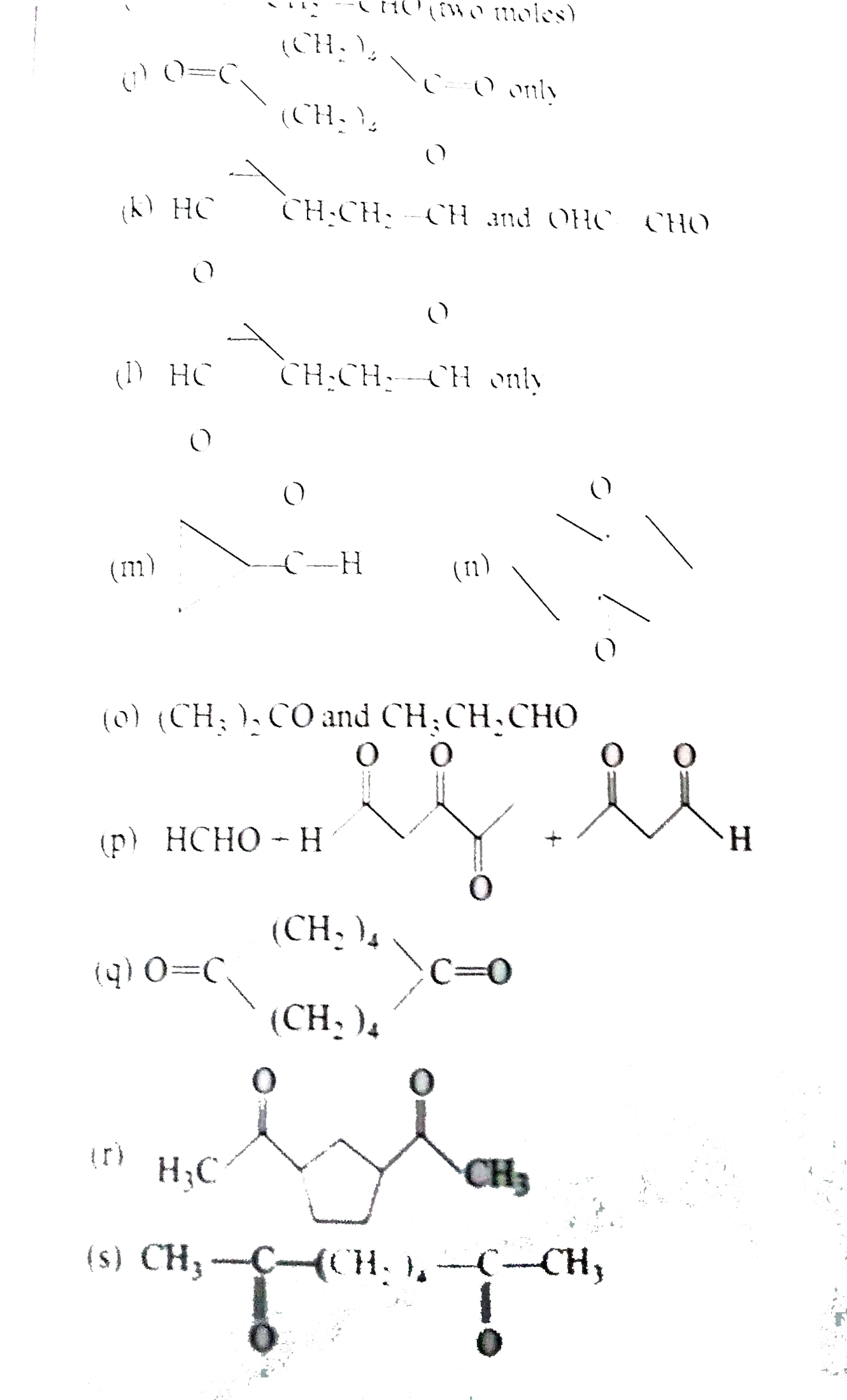
View SolutionWhich of the following compounds can liberate methane when treated with excess of methyl magnesium iodide in dry ether.
(a) CH3−CH2−CH2OH
(b) CH3−CH2−C≡CH
(c) CH3−CH2−COOH
(d) CH3−CH2−CHO
View SolutionSteatement -I: Acrolein containing aldehyde group undergoes aldol condensation with alkali.
Statement -II The duoble bond breaks when treated with NaOH . H2C=CH−CHONaOH−−−→HCHO+CH3CHO
View SolutionTautomerism is possible in
(1) CH3−CHO (3)H−CHO
(2) CH3−CO−CH3 (4)(CH3)3C−CHO
View Solution