Which of the following have identical bond order?
CN−
NO+
O+2
O2−2
The correct Answer is:A, B
To determine which of the given molecules have identical bond orders, we will follow these steps:
Step 1: Identify the total number of electrons in each molecule.
- For CN⁻ (Cyanide ion):
- Carbon (C) has 6 electrons and Nitrogen (N) has 7 electrons. The negative charge adds 1 electron.
- Total electrons = 6 + 7 + 1 = 14 electrons.
- For NO⁺ (Nitric oxide ion):
- Nitrogen (N) has 7 electrons and Oxygen (O) has 8 electrons. The positive charge removes 1 electron.
- Total electrons = 7 + 8 - 1 = 14 electrons.
- For O₂⁺ (Oxygen ion):
- Each Oxygen has 8 electrons, and the positive charge removes 1 electron.
- Total electrons = 8 + 8 - 1 = 15 electrons.
- For O₂⁻ (Oxygen ion):
- Each Oxygen has 8 electrons, and the negative charge adds 1 electron.
- Total electrons = 8 + 8 + 1 = 17 electrons.
Step 2: Determine the bond order using the formula.
The bond order can be calculated using the formula:
Bond Order=12×(Number of bonding electrons−Number of antibonding electrons)
- For CN⁻ (14 electrons):
- The bond order is 3.
- For NO⁺ (14 electrons):
- The bond order is also 3.
- For O₂⁺ (15 electrons):
- The bond order is approximately 2.5.
- For O₂⁻ (17 electrons):
- The bond order is 1.
Step 3: Compare the bond orders.
- CN⁻ has a bond order of 3.
- NO⁺ has a bond order of 3.
- O₂⁺ has a bond order of approximately 2.5.
- O₂⁻ has a bond order of 1.
Conclusion:
The molecules CN⁻ and NO⁺ have identical bond orders of 3.
- For CN⁻ (Cyanide ion):
- Carbon (C) has 6 electrons and Nitrogen (N) has 7 electrons. The negative charge adds 1 electron.
- Total electrons = 6 + 7 + 1 = 14 electrons.
- Nitrogen (N) has 7 electrons and Oxygen (O) has 8 electrons. The positive charge removes 1 electron.
- Total electrons = 7 + 8 - 1 = 14 electrons.
- Each Oxygen has 8 electrons, and the positive charge removes 1 electron.
- Total electrons = 8 + 8 - 1 = 15 electrons.
- Each Oxygen has 8 electrons, and the negative charge adds 1 electron.
- Total electrons = 8 + 8 + 1 = 17 electrons.
The bond order can be calculated using the formula:
Bond Order=12×(Number of bonding electrons−Number of antibonding electrons)
- The bond order is 3.
- The bond order is also 3.
- The bond order is approximately 2.5.
- The bond order is 1.
- CN⁻ has a bond order of 3.
- NO⁺ has a bond order of 3.
- O₂⁺ has a bond order of approximately 2.5.
- O₂⁻ has a bond order of 1.
The molecules CN⁻ and NO⁺ have identical bond orders of 3.
Topper's Solved these Questions
- CHEMICAL BONDING AND MOLECULAR STRUCTUREBOOK - MODERN PUBLICATIONCHAPTER - CHEMICAL BONDING AND MOLECULAR STRUCTUREEXERCISE - NCERT (Exemplar Problems) (Short Answer Type Questions)
21 Videos
- CHEMICAL BONDING AND MOLECULAR STRUCTUREBOOK - MODERN PUBLICATIONCHAPTER - CHEMICAL BONDING AND MOLECULAR STRUCTUREEXERCISE - NCERT (Exemplar Problems) (Matching Type Questions)
5 Videos
- CHEMICAL BONDING AND MOLECULAR STRUCTUREBOOK - MODERN PUBLICATIONCHAPTER - CHEMICAL BONDING AND MOLECULAR STRUCTUREEXERCISE - NCERT (Exemplar Problems) (Multiple Choice Questions (Type-I))
22 Videos
- CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES BOOK - MODERN PUBLICATIONCHAPTER - CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES EXERCISE - UNIT PRACTICE TEST
13 Videos
Similar Questions
Interpret the non-linear shape of H2S molecule and non-planar shape of PCl3 using valence shell electron pair repulsion (VSEPR) theory.
(Atomic number :H=1,P=15,S=16,Cl=17)
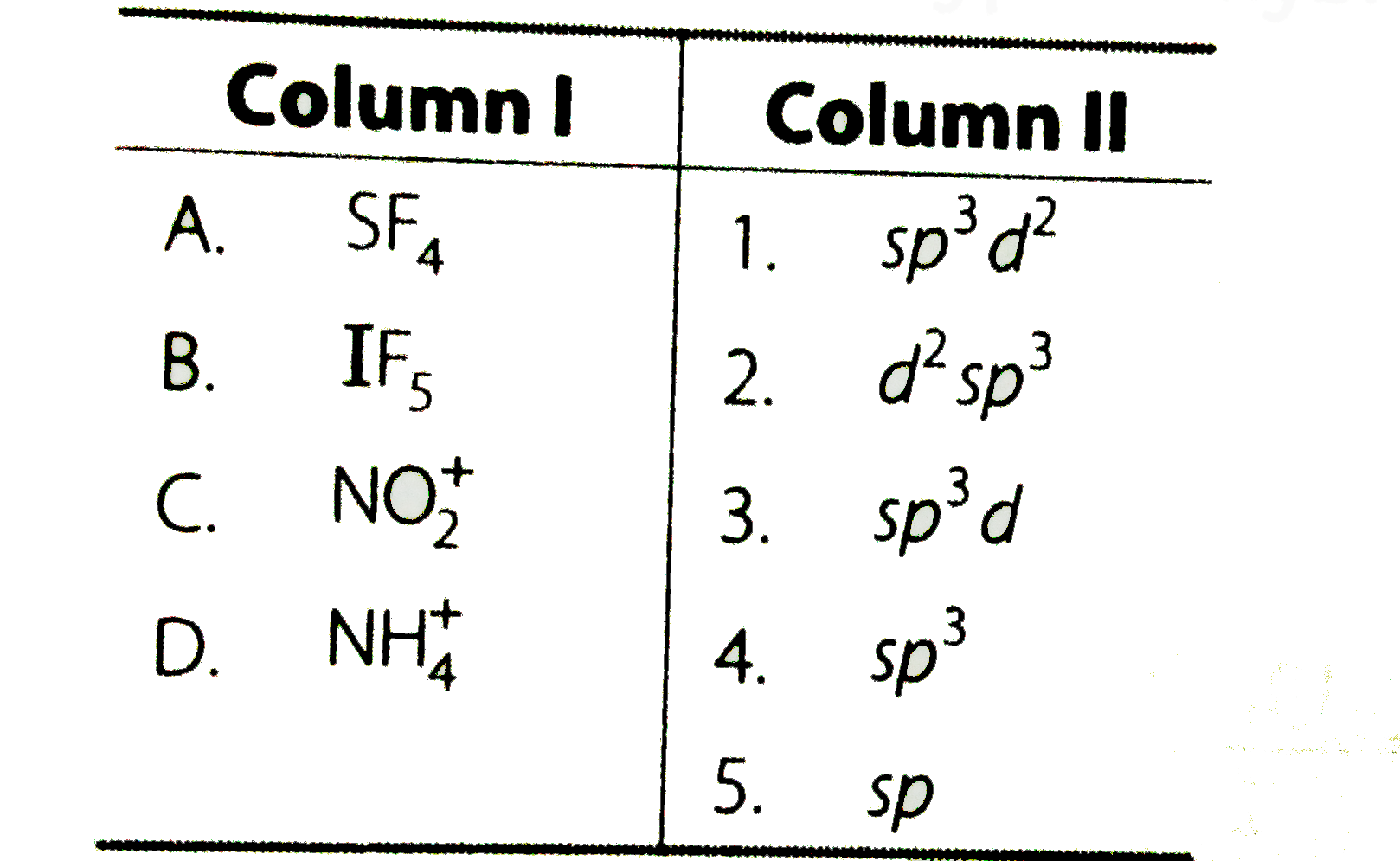
View SolutionMatch the species in Column I with the type of hybrid orbitals in Column II.
View SolutionIsostructural species are those which have the same shape and hybridisation. Among the given identify the isostructural pairs.
View SolutionWhy is the electron gain enthalpy of O or F less than that of S or Cl?
View SolutionWhich of the folowing have identical bond orders ? .
View SolutionWhich of the following pairs have identical value of bond order?
View SolutionIn which of the following paird, the two molecules have identical bond orders :
View SolutionWhich of the following have identical bond order?
View SolutionWhich of the following pairs have identical bond order?
View SolutionWhich of the following pairs have identical bond order:-
View SolutionWhich of the following pairs have identical bond order?
View SolutionWhich of the following have identical bond order
View SolutionIn which of the following pairs the two species have identical bond order?
View SolutionWhich of the following have identical bond order?
View SolutionWhich of the following have identical bond order ?
View Solution

