Which one of the following reactions involves disproportionation ?Solution in Kannada
2H2SO4+Cu→CuSO4+2H2O+SO2
As2O3+3H2S→As2S3+3H2O
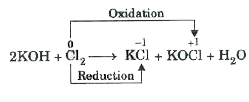
2KOH+Cl2→KCl+KOCl+H2O
Ca3P2+6H2O→3Ca(OH)2+2PH3
The correct Answer is:C
Similar Questions
Which one of the following reaction is an example of disproportionation reaction
View SolutionWhich of the following reactions involve disproportionation ?
View SolutionWhich one of the following is an example of disproportionation reaction?
View SolutionWhich of the following reactions are disproportionation reaction ?
View SolutionWhich one of the following reactions involves disproportionation ?
View Solutionनिम्न अभिक्रियाओं में से कौन-सी असमानुपातन अभिक्रियाएं है?
View SolutionOxidation reaction involves loss of electrons, and reduction reaction involves gain of electrons. The reaction in which a species disproportionates into two oxidation states (lower and higher) is called disproportionation reaction.
Which of the following statements is wrong?
View Solution