ఆదర్శ వాయువు స్థిరోష్ణక మార్పులో △U,w_((adiabatic) ల సంబంధమేమిటి ?
Step by step video & image solution for ఆదర్శ వాయువు స్థిరోష్ణక మార్పులో triangleU,w_((adiabatic) ల సంబంధమేమిటి ? by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
Similar Questions
ఆవర్తన పట్టికలో కర్ణ సంబందం ఉండటానికి గల కారణాలను తెలపండి.
View Solutionవాయు అణువుల మధ్య ఉండే వివిధ రకాల అంతర అణుమూలాలను పేర్కొనండి.
View SolutionThe pressure-volume of varies thermodynamic process is shown in graphs:
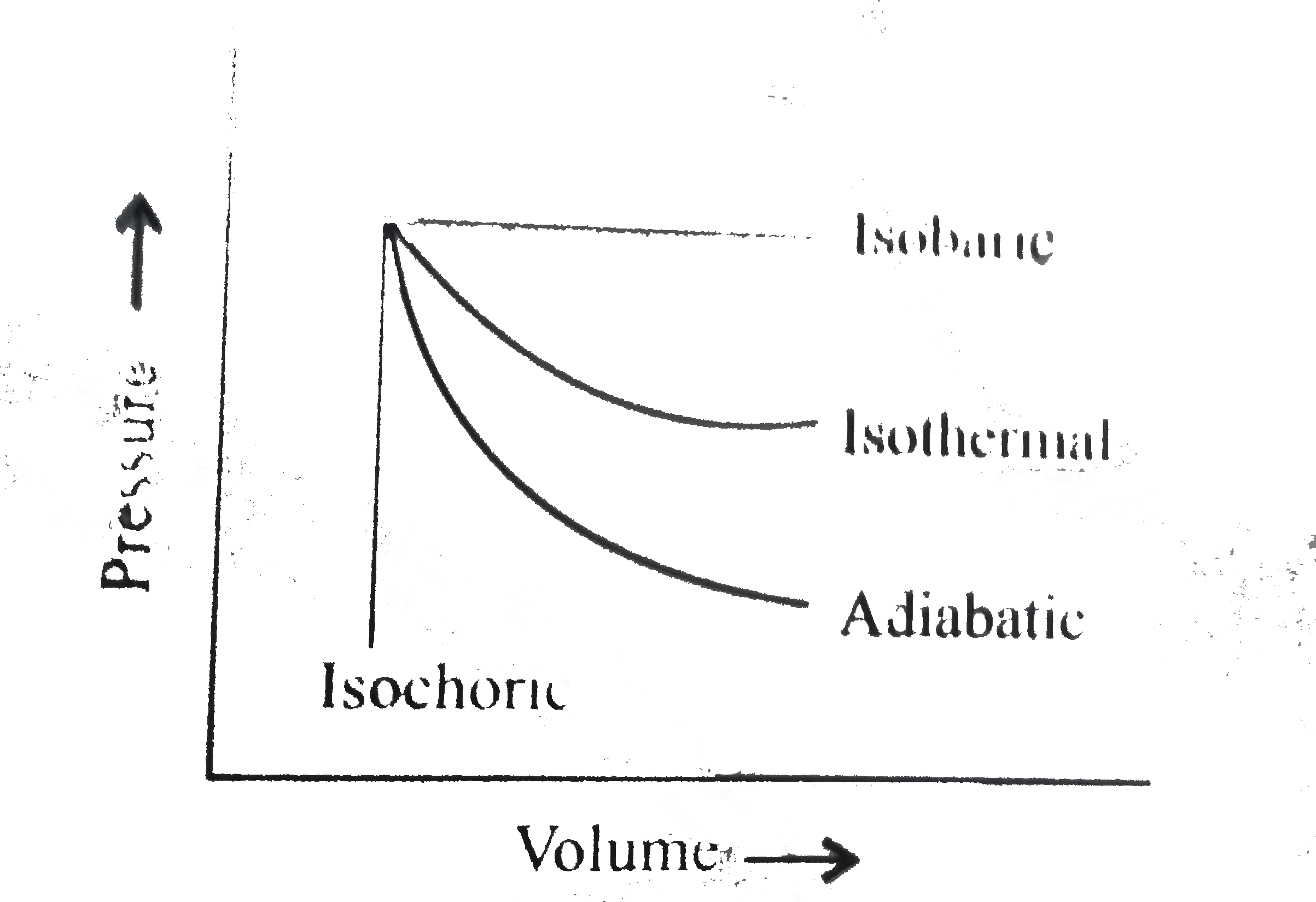
Work is the mole of transference of energy. It has been observed that reversible work done by the system is the maximum obtainable work.
wrev>wirr
The works of isothermal and adiabatic processes are different from each other.
wisothermal reversible=2.303nRTlog10(V2V1)
=2.303nRTlog10(P2P1)
wadiabatic reversible=CV(T1−T2)
If w1,w2,w3 and w4 are work done in isothermal, adiabatic, isobaric, and isochoric reversible processes, respectively then the correct sequence (for expansion) would be
View SolutionIf w1.w2,w3 and w4 are work done in isothermal, adiabatic, isobaric, and isochoric reversible expansion for an ideal gas, respectively, then
View SolutionIf w_(1), w_(2), w_(3) and w_(4) for an ideal gas are magnitude of work done in isothermal, adiabatic, isobaric and isochoric reversible expansion processes, the correct order will be :
View SolutionAssertion : In adiabatic system, ΔU=wad.
Reason : In adiabatic system, no transfer of heat takes place.
View SolutionWith same initial conditions , an ideal gas expands from volume V1 to V2 in three different ways . The work done by the gas is W1 if the process is isothermal , W2 if isobaric and W3 if adiabatic , then
View SolutionIf W1W2,W3andW4 are work done in isothermal, adiabatic, isobaric and isochoric reversible process, the correct order (for expansion) will be
View SolutionBeCl_2(ఘనపదార్థం ) ల నిర్మాణాలను గీయండి.
View Solutionబిందువులు Hమరియు C ల మధ్య దూరమెంత? అల్లాగే బిందువులు A మరియు B ల మధ్య దూరమెంత?
View SolutionIf w_(1), w_(2), w_(3) and w_(4) for an ideal gas are magnitude of work done in isothermal, adiabatic, isobaric and isochoric reversible expansion processes, the correct order will be :
View SolutionAssertion : In adiabatic system, DeltaU=w_(ad.) Reason : In adiabatic system, no transfer of heat takes place.
View SolutionProve that triangleH=triangleU+trianglenRT .
View SolutionIf the system involves gaseous substance, then pressure volume work Wpv=−PextΔV. It has been observed that,
Wrev>Wir.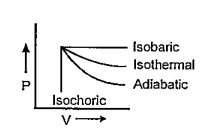
Also the work of isothermal & adiabatic processes are different from each other :
Wisothermalreversib≤=2.303nRTlog(v2v1)
Wadiabaticreversib≤=−Cv(T2−T1)
If W1,W2,W3,W4 are work done in isothermal, adiabatic, isobaric & isochoric reversible processes, then correct sequence would be
View SolutionIf the system involves gaseous substance, then pressure volume work W_(pv) = -P_(ext)DeltaV . It has been observed that, W_(rev) gt W_(ir) . Also the work of isothermal & adiabatic processes are different from each other : W_(isothermal reversible) = 2.303 nRT log(v_2/v_1) W_(adiabatic reversible) = -C_v(T_2-T_1) P-V plot for two gases during adiabatic processes are given as follows : Curves A & B should correspond to respectively
View Solution

