Ideal gas equation for n mole of gasSolution in Tamil
Video Solution
Text Solution
Boyle.s law: For a given gas at low pressure (density) kept in a container of volume Y, experiments revealed the following information.
When the gas is kept at constant temperature, the pressure of the gas is inversely proportional to the volume P∝1V
Charles law: When the gas is kept at constant pressure, the volume of the gas is directly proportional to absolute temperature V∝T.
By combining these two equations we have PV CT. Here C is a positive constant. We can infer that C is proportional to the number of particles in the gas container by considering the following argument. If we take two containers of same type of gas with same volume V, same pressure P and same temperature T, then the gas in each container obeys the above equation. PV = CT. If the two containers of gas is considered as a single system, then the pressure and temperature of this combined system will be same but volume will be twice and number of particles will also be double.
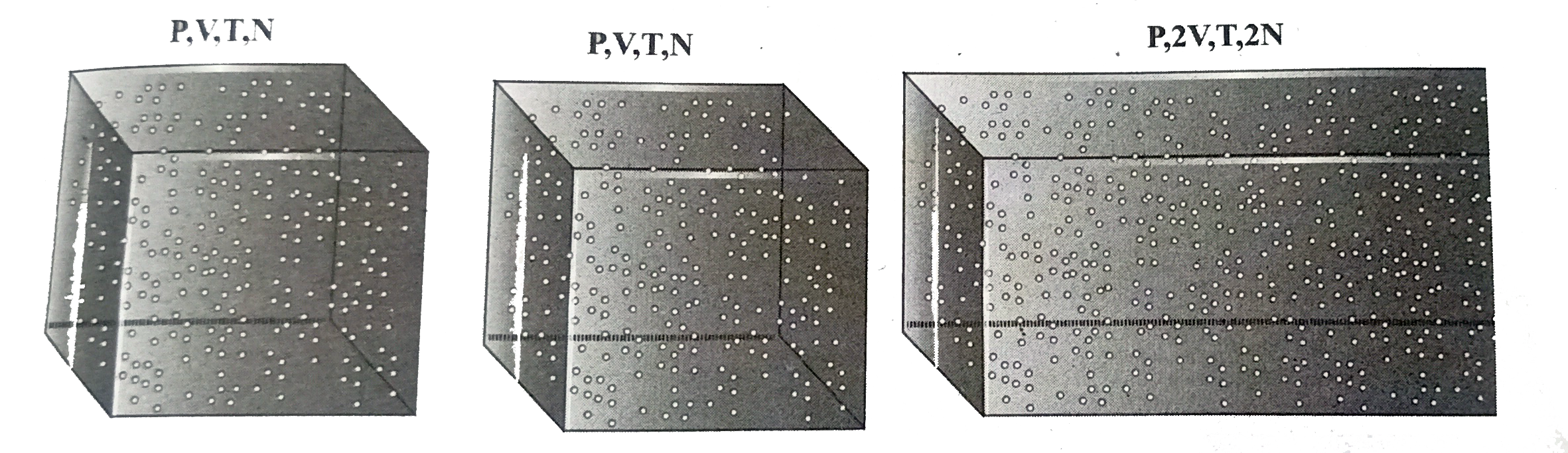
For this combined system, V becomes 2V, so C should also double to match with the ideal gas equation P(2V)T=2C. It implies that C must depend on the number of particles in the gas also should have the dimension of [PVT]=JK−1. So we can write the constant C as k times the umber of particles N.
Here λ is the Boltznnann constant (1.381×1023JK−1) and it is found to be a universal constant, So the idcal gas law can be stated as follows
PV =NKT
The equation (1) can also be expressed in terms of mole. Suppose if a gas contains μ mole of particles then the total number of particles can be written as N=μNA where NA is Avogadro number (6.023×1023mol−1) Substiuting for N from equation (2), the equation (1) becomes PV=μNAkT. Here NAk=R called universal gas constant and its value is 8.314J/mol.K
So the ideal gas law can be written for μ mole of gas as
P=μRT
.(3) This is called the equation of state for an ideal gas. It relates the pressure, volume and temperature of thermodynamic system at equilibrium.
|
Similar Questions
Give the ideal gas equation for n moles of a gas.
View SolutionWrite ideal gas equation for one mole of gas.
View SolutionIdeal gas equation for n mole of gas
View SolutionDerive an equation for n moles of an ideal gas.
View SolutionDistinguish between ideal gas and real gas. Write an equation for one mole of an ideal gas.
View SolutionFor two mole of an ideal gas:
View SolutionFor n moles of an ideal gas, the ideal equation may be written as "_____________" .
View SolutionThe equation of ideal gas for n gram moles is________.
View Solution

