Obtain the law of radioactivity. Law of radioactive decaySolution in Tamil
Video Solution
Text Solution
Law of radioactive decay:
At any instant t, the number of decays per unit time, called rate of decay (dNdt) is proportional to the number of nuclei (N) at the same instant.
dNdt∝N
By introducing a proportionality constant, the relation can be written as
dNdt=−λN ...(1)
Here proportionality constant λ is called decay constant which is different for different radioactive sample and the negative sign in the equation implies that the N is decreasing with time. By rewriting the equation (1), we get
dN=−λNdt ...(2)
Here dN represents the number of nuclei decaying in the time interval dt. Let us assume that at time t = 0 s, the number of nuclei present in the radioactive sample is N0 . By integrating the equation (2), we can calculate the number of undecayed nuclei N at any time t. From equation (2), we get
dNN=−λdt ...(3)
∫NN0dNN=−∫10λdt
In[NN0]=−λt
Taking exponentials on both sides, we get
N=N0e−λt...(4)
Equation (4) is called the law of radioactive decay. Here N denotes the number of undecayed nuclei present at any time t and No denotes the number of nuclei at initial time t = 0. Note that the number of atoms is decreasing exponentially over the time. This implies that the time taken for all the radioactive nuclei to decay will be infinite. Equation (4) is plotted.
We can also define another useful quantity called activity (R) or decay rate which is the number of nuclei decayed per second and it is denoted as R=∣∣∣dNdt∣∣∣
Note: that activity R is a positive quantity. From equation (4), we get
R=∣∣∣dNdt∣∣∣=λN0e−λt...(5)
R=R0e−λt...(6)
R0=λN0
The equation (6) is also equivalent to radioactive law of decay. Here Ro is the activity of the sample at t = 0 and R is the activity of the sample at any time t. From equation (6), activity also shows exponential decay behavior. The activity R also can be expressed in terms of number of undecaycd atoms present at any time t. From equation (6), since N=N0e−λt we write R=λN...(7)
Equation (4) implies that the activity at any time t is equal to the product of decay constant and number of undecaycd nuclei at the same time t. Since N decreases over time, R also decreases.
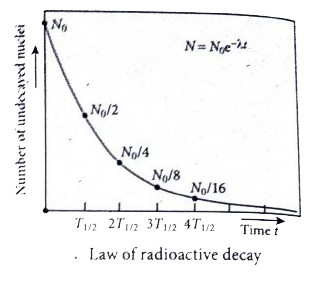
|
Similar Questions
Describe the laws of radioactive decay. Derive equation of radioactive decay using these laws.
View Solutionरेडियोसक्रिय क्या है ? रेडियोसक्रिय क्षय का नियम बताएँ । यह दर्शाएँ कि रेडियोसक्रिय क्षय प्रकृति का है।
View SolutionStability Curve and Laws of Radioactive Decay
View SolutionState the law of radioactive decay. Show that radioactive decay is exponential in nature.
View SolutionState the law of radioactive decay. Sketch a graph to illustrate radioactive decay.
View SolutionState the law of radioactive decay.
View SolutionState radioactive decay law.
View SolutionState radioactive decay law.
View Solution
