Complete the following reactions.
(a)PCI3+Cl2→?
(b)SiO2+?→CaSiO3
(C )2HgOΔ−−→?+O2
(d)CaCO3Δ−−→?+CO2
(e ) Ca(OH)2CO2−−→?+H2O
(a)PCl3+Cl2→PCl5
(b)SiO2+CaO→CaSiO3
(c )2HgOΔ−−→2Hg+O2
(d)CaCO3Δ−−→CaO+CO2
( e)Ca(OH)2CO2−−→→CaCO3+H2O
Topper's Solved these Questions
- TYPES OF CHEMICAL REACTIONSBOOK - FULL MARKSCHAPTER - TYPES OF CHEMICAL REACTIONSEXERCISE - Additional Problems
6 Videos
- TYPES OF CHEMICAL REACTIONSBOOK - FULL MARKSCHAPTER - TYPES OF CHEMICAL REACTIONSEXERCISE - Additional question solved (Long answer questions.)
6 Videos
- SOLUTIONSBOOK - FULL MARKSCHAPTER - SOLUTIONSEXERCISE - ACTIVITY
1 Video
Similar Questions
Knowledge Check
What are A––,B––,C–– in the following reactions ?
(I) (CH3CO)2CaΔA––
II.CH3CO2HHI−−→RedPb–
(III) 2CH3CO2HP4O10−−−→C––AA−C2H6, B−CH3COCH3,C−(CH3CO)2OBA−(CH3CO)2O,B−C2H6,C−CH3COCH3CA−CH3COCH3,B−C2H6,C−(CH3CO)2ODA−CH3COCH3,B−(CH3CO)2O,C−C2H6The product D in the following reaction is ___________.
CH3ClKCN−−→(A)H2O−−→(B)NH3−−→(C)Δ−−→(D)ACH3CH2NH2BCH3CNCHCONH2DCH3CONH2
Complete the following reactions:
a. PhCHO+CH3NO2ΘOH−−→Δ(A)
b. Me2CO+CHCI3ΘOH−−→(B)
c. PhCHO+CH3C≡NNΘH2−−→(C)
d. Me2CO+ ,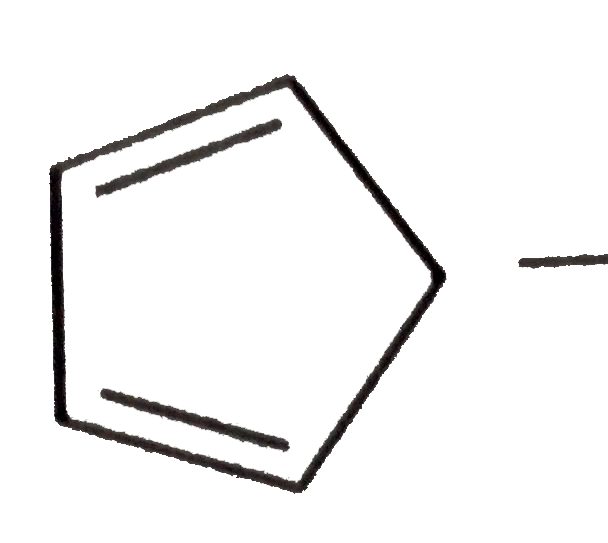 , ΘOH−−→Δ(D)
, ΘOH−−→Δ(D)
e. PhCHO+ ,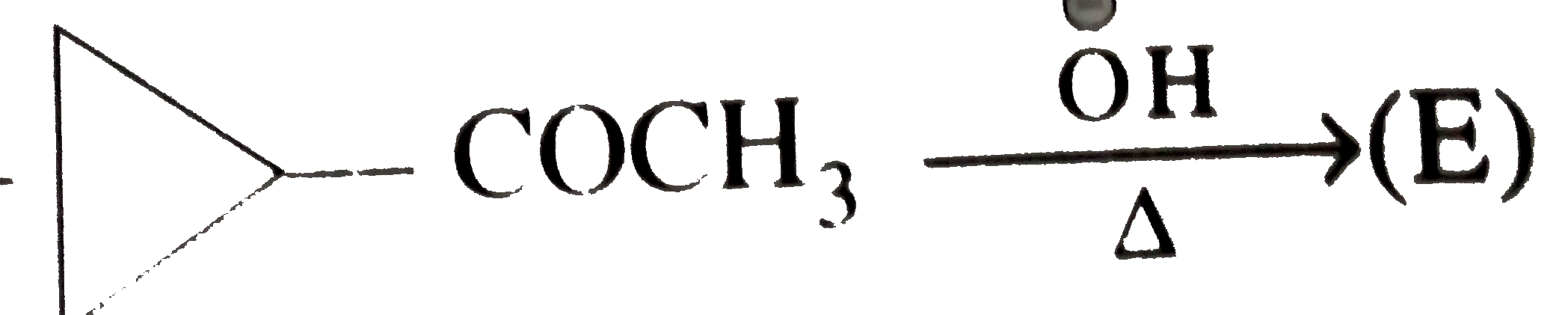
f.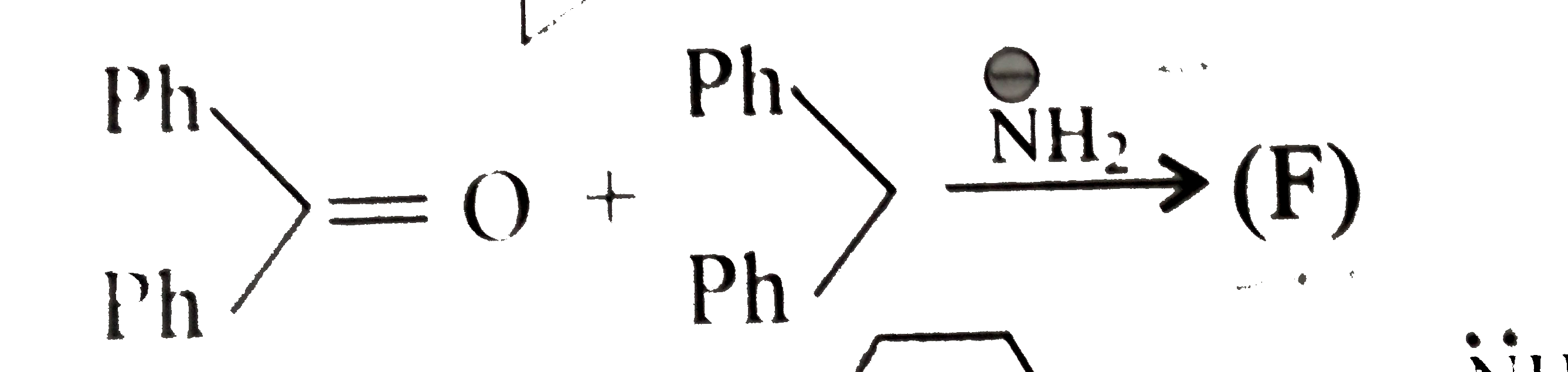
g. PhCHO+ ,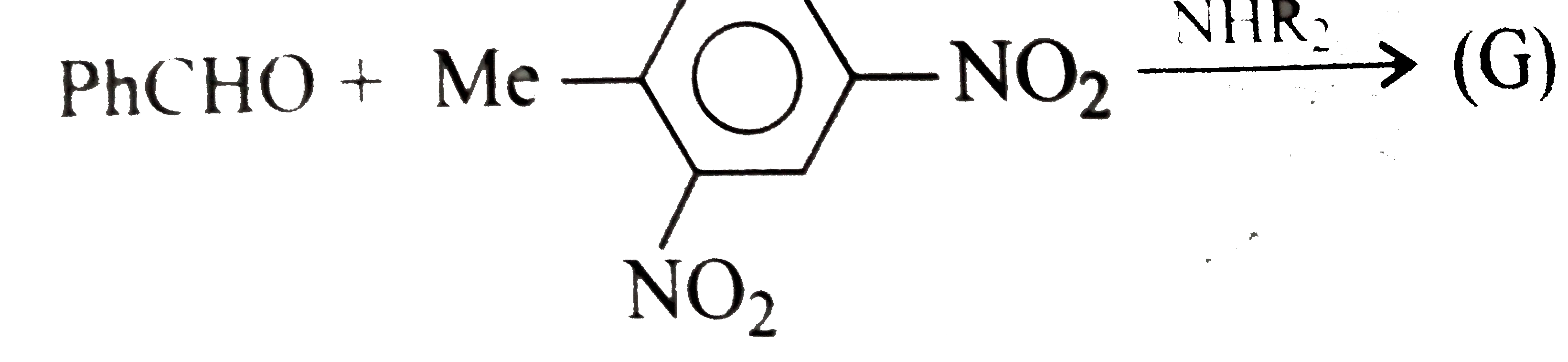
View SolutionFind the total number of feasible reaction which would yeild free metal.
(a) HgS+O2600∘C−−−→ΔHg+SO2
(b) HgS+2HgOΔ−−→3Hg+SO2
(c ) CuSO4+3CO→3Cu+2SO2
(d) Fe2O3+3COΔ−−→2Fe+3CO2
(e) Fe2O3+3CΔ−−→2Fe+3CO
(f) ZnSO4+2CΔ−−→Zn+SO2+2CO
(g) 4Cu2O+CH4→8Cu+2H2O+CO2
(h) 2Na[Au(CN)2]+Zn→Na2[Zn(CN)4]+2Au
View SolutionComplete the following reactions :
(a) AI(OH)−4+CO2→?+?+?
(b) Ag++Na2S2O3→?+?
(c) MnO−4+H2SΔ−−→?+?
View SolutionComplete the following reactions :
(a) Al(OH)−4+CO2→?+?+?
(b) Ag++Na2S2O3→?+?
(c) MnO−4+H2SΔ−−→?+?
View SolutionComplete the following reactions:
(i) CH3−CH3∣C∣Cl−CH2CH3alcKOH−−−−→ΔAO3−−−−−→Zn/H2O(B+C)
(ii) CH3ICH−CH3alcKCN−−−−→A(i)SnCl2/HCl−−−−−−−−−−−−→(ii)H2O.OH.H2O2B
(iii) HCNHCl/AlCl3−−−−−−→A(i)C6H6Δ−−−−−−−−−−→(ii)(H2O)/H−B
(iv)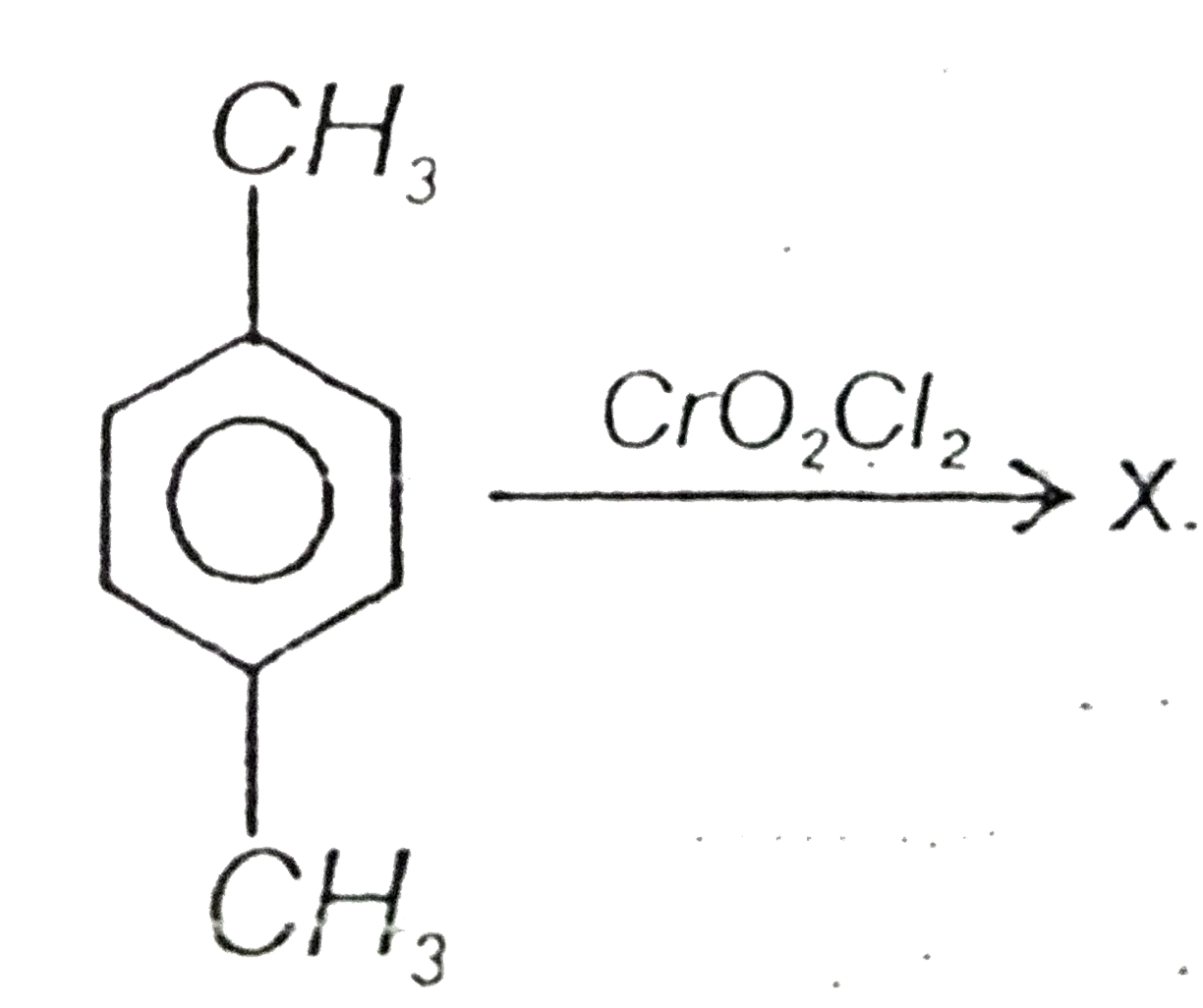
View SolutionComplete the following reactions:
(i) CH3−CH3∣C∣Cl−CH2CH3alcKOH−−−−→ΔAO3−−−−−→Zn/H2O(B+C)
(ii) CH3ICH−CH3alcKCN−−−−→A(i)SnCl2/HCl−−−−−−−−−−−−→(ii)H2O.OH.H2O2B
(iii) HCNHCl/AlCl3−−−−−−→A(i)C6H6Δ−−−−−−−−−−→(ii)(H2O)/H−B
(iv)
View Solution
