Enthalpy diagram for a particular reaction is given in Fig.6.Is it possible to decide spontaneity of a reaction from given diagram.Explain.

It is not possible to decide spontaneity of the reaction from the given diagram.Enthalpy change is one of the contributing factors in predicting the spontaneity of the reaction.But it is not the only factor.The another factor which helps to decide the spontaneity of a reaction is entropy factor. The resultant of these two factors known as Gibbs free energy is the main factor which decide the spontaneity of a reaction.
Topper's Solved these Questions
Similar Questions
Match the following
AB(I) Adiabatic processHeatII Isolated system(b) At constant volume(iii) Isothermal change(c) First law of thermodynamicsIv Path function (d) No exchange of energy and matter(v) State Function(e) No transfer of heat(iv)ΔU=q(f) Constant temperatureVii) Law of conservation of energy (g) Internal energyviii Reversible process(h)Pext=0ix Free expansion (i) At constant pressure x)ΔH=q(j) Infinitely slow process which proceeds through a series of equilibrium states (xi) Intensive property(k) Entropy(xii) Extensive property(l) Pressure(m) Specific heat
View SolutionEnthalpy diagram for a particular reaction is given in figure. Is it possible to decide spontaneity of a reaction from given diagram. Explain
View Solutionदिया गया एन्थैल्पी चित्र, निम्न में से कौन-सी अभिक्रिया प्रदर्शित करता है?
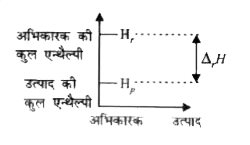
View SolutionEnthalpy diagram for a particular reaction is given in figure. Is it possible to decide spontaneity of a reaction from given diagram. Explain.
View SolutionThe given enthalpy diagram reperesents which of the following reactions ?
View SolutionSpontaneity of a chemical reaction is decided by the negative change in _________ .
View SolutionQuestions given below are based on the given diagram for extractive metallurgy.
(iv) At 1100∘C, which reaction is spontaneous to a maximum extent ?
View SolutionEnthalpy of a reaction is given as:
View Solution

