SN1/SN2 proceeds through the formation of a carbocation.
SN1
Similar Questions
Aliphatic nucleophilic substitution is mainly of two type SN1 and SN2.SN2 reaction proceed with strong nucleophile in polar aprotic solvent. 3∘ halides do not give SN2 reaction. Inverted products are obtained in this reaction and mechanism of reaction occurs through the formation of transition state.
SN1 reaction proceed through the formation of carbocation in polar aprotic solvent. Solvent itself acts as nucleophile in this reaction. Racemization takes place in SN1 reaction.
Which one of the following will give SN2 reaction?
View SolutionAliphatic nucleophilic substitution is mainly of two type SN1 and SN2.SN2 reaction proceed with strong nucleophile in polar aprotic solvent. 3∘ halides do not give SN2 reaction. Inverted products are obtained in this reaction and mechanism of reaction occurs through the formation of transition state.
SN1 reaction proceed through the formation of carbocation in polar aprotic solvent. Solvent itself acts as nucleophile in this reaction. Racemization takes place in SN1 reaction.
Which one of the following will give racemised product in C2H5OH?
View Solution
Knowledge Check
Assertion (A) Hydrolysis of (-)-2-bromooctane proceeds with inversion of configuration
Reason (R) This reaction proceeds through the formation of a carbocation.AAssertion and reason both are correct and reason is correct explanation of assertionBAssertion and reason both are wrong statementsCAssertion is correct but reason is wrong statementDAssertion is wrong but reason is correct statement.SN2 mechanism proceeds through formation of :
ACarbonium ionBTransition stateCFree radicalDCarbanionAliphatic nucleophilic substitution is mainly of two type SN1 and SN2.SN2 reaction proceed with strong nucleophile in polar aprotic solvent. 3∘ halides do not give SN2 reaction. Inverted products are obtained in this reaction and mechanism of reaction occurs through the formation of transition state.
SN1 reaction proceed through the formation of carbocation in polar aprotic solvent. Solvent itself acts as nucleophile in this reaction. Racemization takes place in SN1 reaction.
Which of the following compounds will give SN1 reaction ?APh−CH3∣C∣Cl−CH2CH3BH3C−ClCH3C−CH2−BrD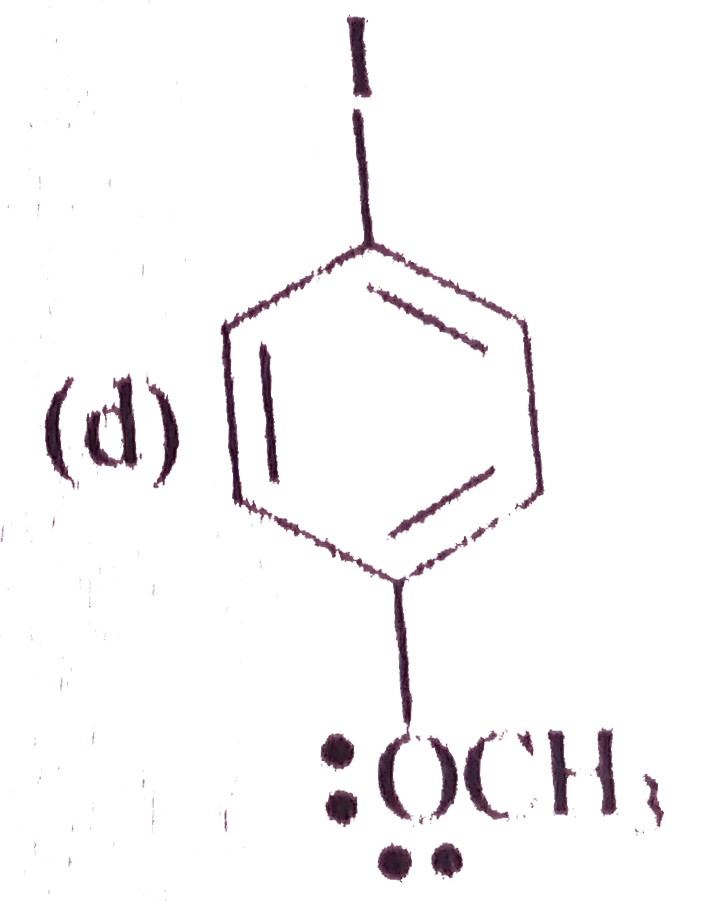
Assertion: Hydrolysis of (-)-2- bromooctane proceeds with inversion of configuration.
Reason: This reaction prioceeds through the formation of a carbocation.
View SolutionAssertion: Hydrolysis of (-)-2- bromooctane proceeds with inversion of configuration.
Reason: This reaction prioceeds through the formation of a carbocation.
View SolutionAssertion: Hydrolysis of (-)-2- bromooctane proceeds with inversion of configuration.
Reason: This reaction prioceeds through the formation of a carbocation.
View SolutionSN2 mechanism proceeds through the formation of
View SolutionAssertion: tert-butylbromide undergoes SN1 nucleophilic substitution readily than n-butyl bromide.
Reason: It proceeds by the formation of stable carbocation.
View Solution

