The compound having longest C-Cl bond is
A
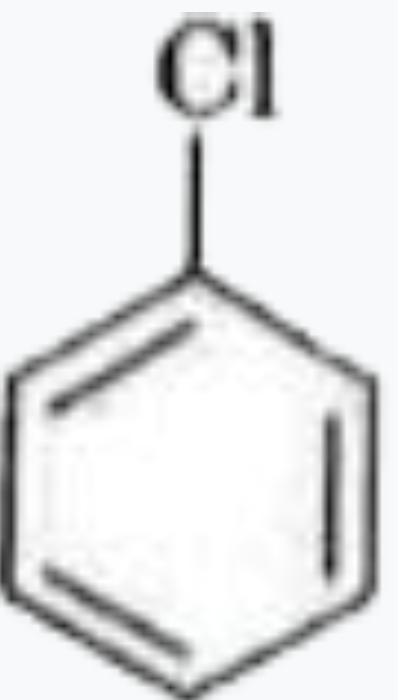
B
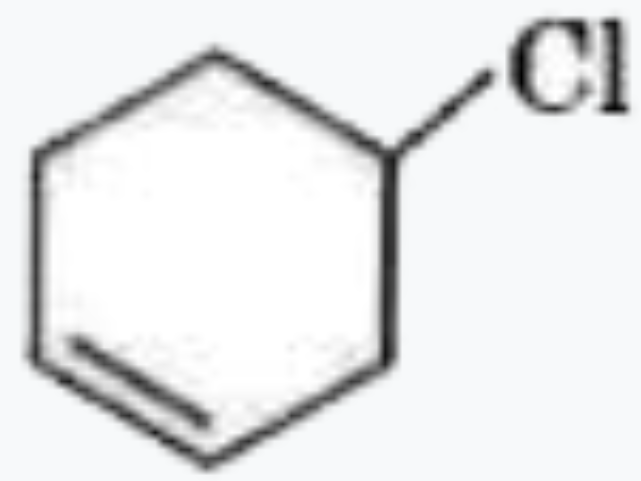
C
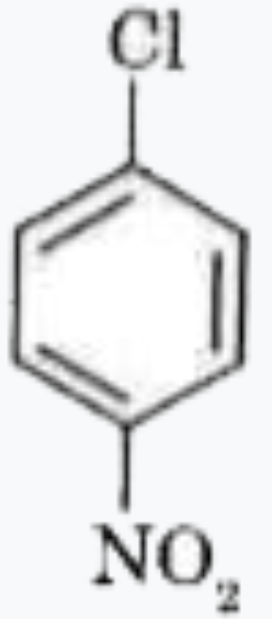
D
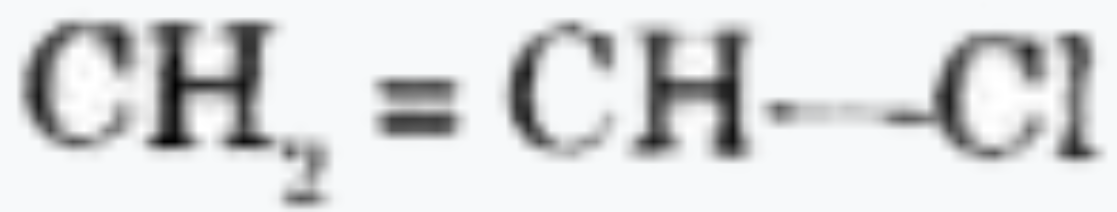
Text Solution
The correct Answer is:B
In 4-chloroyelohexane bond length is largest because there is no resonance. In other compounds, formation of double bond takes place between C-Cl bond because of resonance.

|
Topper's Solved these Questions
- HALOALKANES AND HALOARENESBook:MODERN PUBLICATIONChapter:HALOALKANES AND HALOARENESExercise:C. MULTIPLE CHOICE QUESTIONS (with more than one correct answers)
Explore 10 Videos
- HALOALKANES AND HALOARENESBook:MODERN PUBLICATIONChapter:HALOALKANES AND HALOARENESExercise:D. MULTIPLE CHOICE QUESTIONS (based on the given passage / comprehension)
Explore 9 Videos
- HALOALKANES AND HALOARENESBook:MODERN PUBLICATIONChapter:HALOALKANES AND HALOARENESExercise:A. MULTIPLE CHOICE QUESTIONS (with only one correct answer)
Explore 35 Videos
- EQUILIBRIUMBook:MODERN PUBLICATIONChapter:EQUILIBRIUMExercise:Unit Practice Test
Explore 13 Videos
- HYDROCARBONSBook:MODERN PUBLICATIONChapter:HYDROCARBONSExercise:UNIT PRACTICE TEST
Explore 15 Videos
Similar Questions
Knowledge Check
Which of the following has longest C-O bond?
A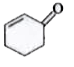 B
B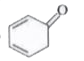 C
C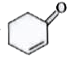 D
D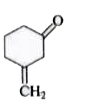
Which of the following has longest C-O bond
A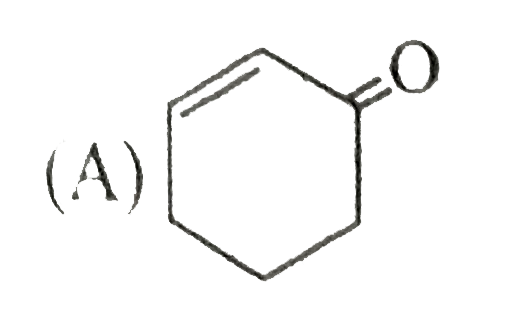 B
B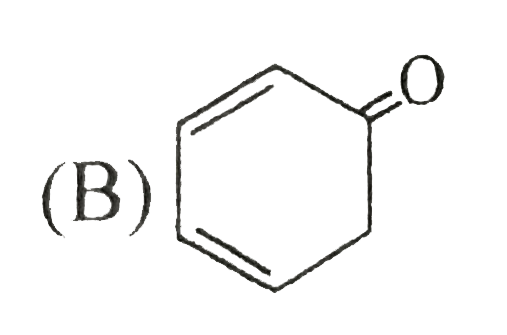 C
C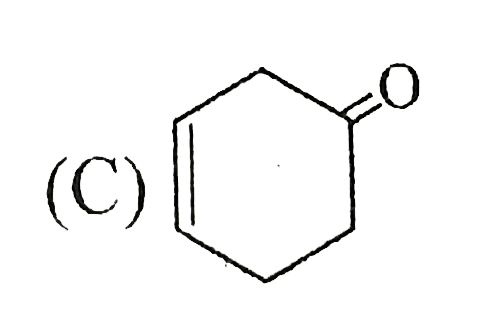 D
D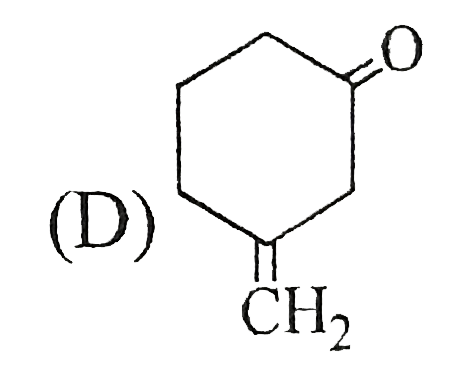
Which of the following has longest C-O bond?
A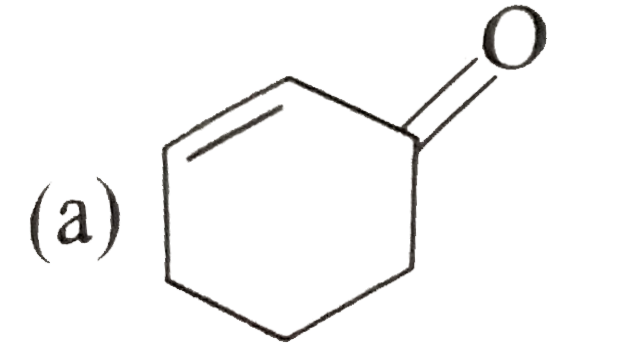 B
B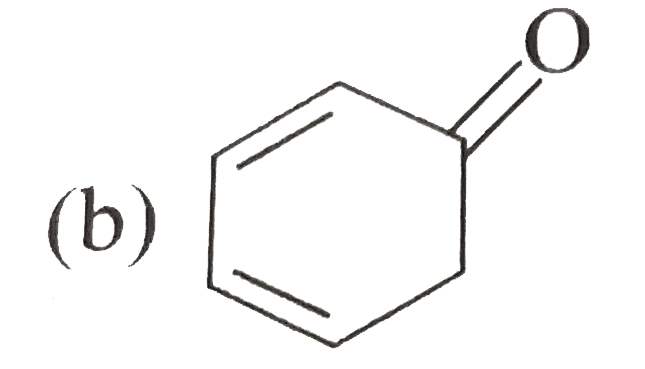 C
C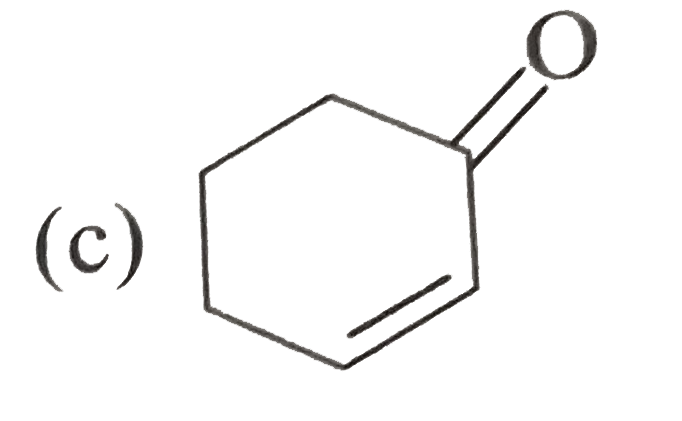 D
D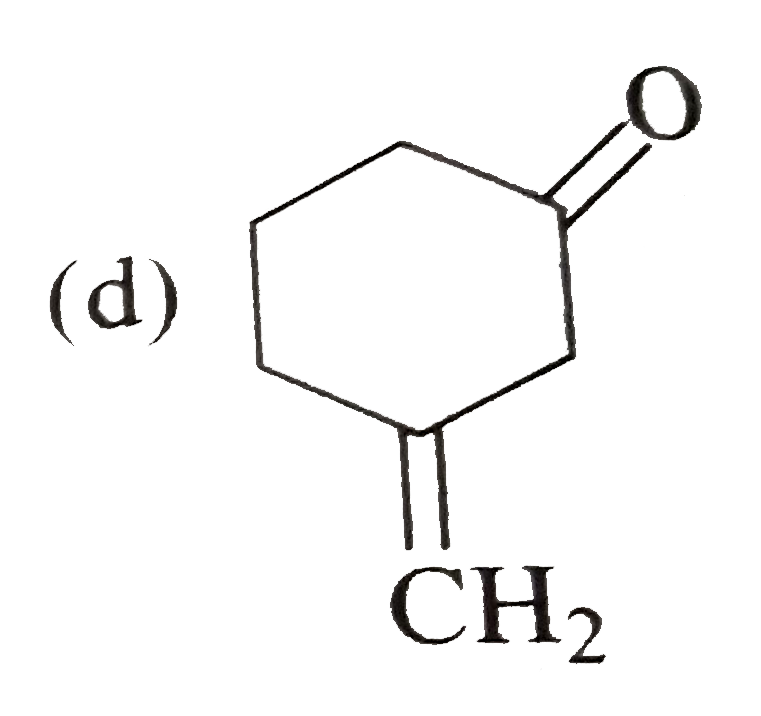
Which of the following compounds will have the longest C = C bonds?
View SolutionWhich of the following has the longest C-O bond length? (C-O bond length of CO is 1.128 A)
View SolutionWhich of the following has longest C−O bond length? (Free C−O bond length in CO is 1.128Å).
View SolutionIn which of the following compounds, the C-Cl bond ionisation shall give most stable carbonium ion?
View SolutionIn which of the following compounds, the C-Cl bond ionisation shall give most stable carbonium ion?
View Solution

