A small amount of ethyl alcohol is usually added to chloroform bottles. Why?
Step-by-Step Solution:
1. Understanding Chloroform's Reactivity: Chloroform (CHCl3) is a volatile organic compound that can react with oxygen in the presence of sunlight. This reaction can lead to the formation of harmful byproducts.
2. Formation of Phosgene: One of the toxic byproducts formed when chloroform reacts with oxygen is phosgene (COCl2). Phosgene is a colorless gas that is highly toxic and poses serious health risks.
3. Role of Ethyl Alcohol: To mitigate the formation of phosgene, a small amount of ethyl alcohol (C2H5OH) is added to chloroform bottles. Ethyl alcohol acts as a protective agent.
4. Reaction with Phosgene: When ethyl alcohol is present, it can react with the phosgene that may form. This reaction converts phosgene into diethyl carbonate (C2H5CO3), which is a harmless compound.
5. Balancing the Reaction: The reaction can be represented as follows:
2C2H5OH+COCl2→2C2H5CO3+2HCl
This shows that ethyl alcohol effectively neutralizes the toxic phosgene gas.
6. Conclusion: Therefore, the addition of ethyl alcohol to chloroform bottles serves to prevent the formation of toxic phosgene and ensures safety by converting it to a harmless compound.
2C2H5OH+COCl2→2C2H5CO3+2HCl
This shows that ethyl alcohol effectively neutralizes the toxic phosgene gas.
Similar Questions
Knowledge Check
An aqueous solution of ethyl alcohol is
Aneutral in natureBacidic in natureCamphotericDbasic in natureFind density of ethyl alcohol
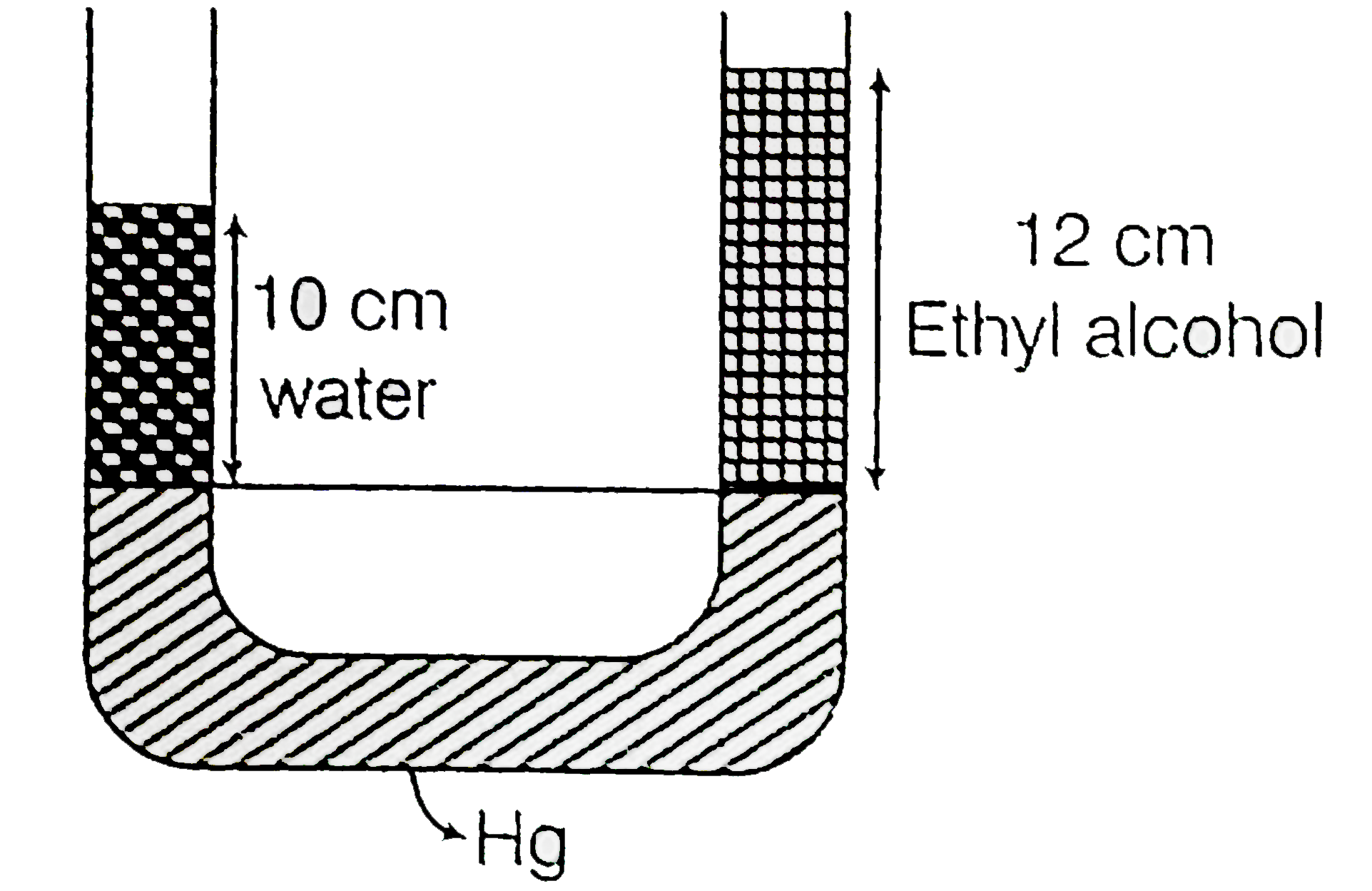 A0.83gmcm3B0.5gmcm3C1.83gmcm3D0.12gmcm3
A0.83gmcm3B0.5gmcm3C1.83gmcm3D0.12gmcm31% pure ethyl alcohol is added to the chloroform used in hospitals because:
AIt enhances the oxidation of chloroformBThe poisonous gas phosgene is not formedCEthyl alcohol converts phosgene into into diethyl carbonate non-toxic saltDIt is helpful in producing unconsciousness
Ethylamine to ethyl alcohol
View SolutionSmall quantity of alcohol is added to chloroform to remove____formed as a result of exposure to air and light.
View SolutionEthyl alcohol is used as a preservative for chloroform because it :
View SolutionEthyl alcohol is used as a preservative for chloroform because it :
View SolutionEthyl alcohol is used as a preservative for chloroform because it :
View Solution

