Chlorobenzene gives a white precipitate with alcoholic silver nitrate solution.
To determine whether chlorobenzene gives a white precipitate with alcoholic silver nitrate solution, we need to analyze the reaction step by step.
Step 1: Understanding the Reactants
Chlorobenzene (C6H5Cl) is an aromatic compound where a chlorine atom is bonded to a benzene ring. Alcoholic silver nitrate (AgNO3 in ethanol) is a reagent often used to test for the presence of halides.
Hint: Identify the structure of chlorobenzene and the components of alcoholic silver nitrate.
Step 2: Reaction Mechanism
When chlorobenzene is mixed with alcoholic silver nitrate, we expect a reaction that would typically involve the displacement of the chlorine atom by the silver ion (Ag+), leading to the formation of silver chloride (AgCl), which is a white precipitate.
Hint: Consider the type of bond present in chlorobenzene and how it might affect reactivity.
Step 3: Analyzing the Bonding
In chlorobenzene, the carbon-chlorine bond has partial double bond character due to resonance. The lone pair of electrons on the chlorine atom participates in resonance with the aromatic system, making the C-Cl bond stronger and less reactive.
Hint: Recall the concept of resonance and how it stabilizes the molecule.
Step 4: Conclusion on Reactivity
Due to the resonance stabilization in chlorobenzene, the C-Cl bond does not easily break to allow for the formation of AgCl. Therefore, chlorobenzene does not react with alcoholic silver nitrate to produce a white precipitate.
Hint: Think about how the stability of the molecule influences its reactivity.
Final Answer
Chlorobenzene does not give a white precipitate with alcoholic silver nitrate solution. The statement is false.
Chlorobenzene (C6H5Cl) is an aromatic compound where a chlorine atom is bonded to a benzene ring. Alcoholic silver nitrate (AgNO3 in ethanol) is a reagent often used to test for the presence of halides.
When chlorobenzene is mixed with alcoholic silver nitrate, we expect a reaction that would typically involve the displacement of the chlorine atom by the silver ion (Ag+), leading to the formation of silver chloride (AgCl), which is a white precipitate.
In chlorobenzene, the carbon-chlorine bond has partial double bond character due to resonance. The lone pair of electrons on the chlorine atom participates in resonance with the aromatic system, making the C-Cl bond stronger and less reactive.
Due to the resonance stabilization in chlorobenzene, the C-Cl bond does not easily break to allow for the formation of AgCl. Therefore, chlorobenzene does not react with alcoholic silver nitrate to produce a white precipitate.
Chlorobenzene does not give a white precipitate with alcoholic silver nitrate solution. The statement is false.
Similar Questions
Knowledge Check
Which one of the following will not give white precipitate with ammonical silver nitrate solution
ACH3=C∘C−CH3BCH3−CH∣CH3−C≡−CH3CCH3−CH2−CH=CH2Dall of theseWhich of the following compound gives white precipitate with silver nitrate solution?
ANaCIBNa2CO3CNa2SO3DNa2S2O3Which of the following will not give white precipitate with ammoniacal silver nitrate solution ?
ACH3−C≡C−CH3B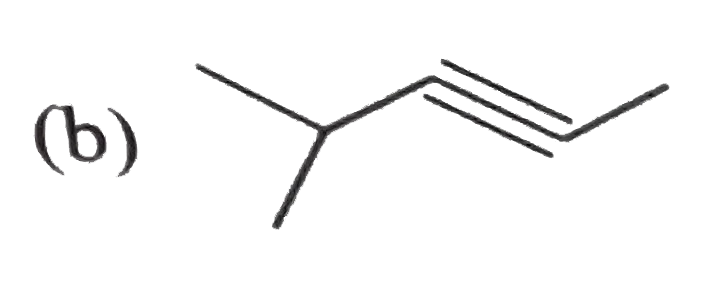 C
C D
D
Which one of the following will not goves white precipitate with ammonical silver nitrate solution ?
View SolutionWhich one of the following does not give white precipitate with acidified silver nitrate solution?
View SolutionH3PO4Δ−−→XΔ−−→Y gives a white precipitate with silver nitrate solution.
X is a
View SolutionH3PO4Δ−−→XΔ−−→Y gives a white precipitate with silver nitrate solution.
Y can form polyanions. The one that represents the polyanion is:
View SolutionSilver nitrate solution when added to a colourless aqueous solution E forms a white precipitate which dissolves in excess of E. if the white precipitate is heated with water it turs black and the supernatant solution gives a white precipitate with acidified barium nitrate solution. Therefore, E is:
View Solution

