Which of the following is not a polyhalogen compound?
Chloroform
Freon
Carbon tetrachloride
Chlorobenzene
The correct Answer is:D
To determine which of the given options is not a polyhalogen compound, we first need to understand what a polyhalogen compound is. A polyhalogen compound is defined as a compound that contains more than one halogen atom.
Let's analyze each option step by step:
Step 1: Analyze Option A - Chloroform (CHCl₃)
- Chloroform has the chemical formula CHCl₃.
- It contains one carbon atom (C), one hydrogen atom (H), and three chlorine atoms (Cl).
- Since there are three chlorine atoms, this compound has more than one halogen.
- Conclusion: Chloroform is a polyhalogen compound.
Step 2: Analyze Option B - Freon
- Freon is a name for a class of compounds, but let's consider a common example, such as Freon-12 (CCl₂F₂).
- Freon-12 contains two chlorine atoms (Cl) and two fluorine atoms (F).
- Since it contains more than one halogen (Cl and F), it qualifies as a polyhalogen compound.
- Conclusion: Freon is a polyhalogen compound.
Step 3: Analyze Option C - Another Freon Example (CCl₃F)
- If we consider another Freon example, such as CCl₃F, it contains three chlorine atoms and one fluorine atom.
- Again, this compound has more than one halogen.
- Conclusion: This Freon compound is also a polyhalogen compound.
Step 4: Analyze Option D - Chlorobenzene (C₆H₅Cl)
- Chlorobenzene has the chemical formula C₆H₅Cl.
- It contains one chlorine atom (Cl) attached to a benzene ring.
- Since there is only one halogen atom present, it does not meet the criteria for a polyhalogen compound.
- Conclusion: Chlorobenzene is not a polyhalogen compound.
Final Answer
Based on the analysis, the compound that is not a polyhalogen compound is Option D - Chlorobenzene (C₆H₅Cl).
---
- Chloroform has the chemical formula CHCl₃.
- It contains one carbon atom (C), one hydrogen atom (H), and three chlorine atoms (Cl).
- Since there are three chlorine atoms, this compound has more than one halogen.
- Conclusion: Chloroform is a polyhalogen compound.
- Freon is a name for a class of compounds, but let's consider a common example, such as Freon-12 (CCl₂F₂).
- Freon-12 contains two chlorine atoms (Cl) and two fluorine atoms (F).
- Since it contains more than one halogen (Cl and F), it qualifies as a polyhalogen compound.
- Conclusion: Freon is a polyhalogen compound.
- If we consider another Freon example, such as CCl₃F, it contains three chlorine atoms and one fluorine atom.
- Again, this compound has more than one halogen.
- Conclusion: This Freon compound is also a polyhalogen compound.
- Chlorobenzene has the chemical formula C₆H₅Cl.
- It contains one chlorine atom (Cl) attached to a benzene ring.
- Since there is only one halogen atom present, it does not meet the criteria for a polyhalogen compound.
- Conclusion: Chlorobenzene is not a polyhalogen compound.
Based on the analysis, the compound that is not a polyhalogen compound is Option D - Chlorobenzene (C₆H₅Cl).
Topper's Solved these Questions
- HALOALKANES AND HALOARENESBook:MODERN PUBLICATIONChapter:HALOALKANES AND HALOARENESExercise:REVISION EXERCISE (Objective Questions) (Passage based questions)
Explore 10 Videos
- HALOALKANES AND HALOARENESBook:MODERN PUBLICATIONChapter:HALOALKANES AND HALOARENESExercise:REVISION EXERCISE (Objective Questions) (Assertion Reason questions)
Explore 8 Videos
- HALOALKANES AND HALOARENESBook:MODERN PUBLICATIONChapter:HALOALKANES AND HALOARENESExercise:MEMORY TEST (C. Choose the correct alternative)
Explore 12 Videos
- GENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTSBook:MODERN PUBLICATIONChapter:GENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTSExercise:COMPETION FILE (Integer Type Numerical Value Type Questions)
Explore 5 Videos
- ORGANIC COMPOUNDS CONTAINING NITROGEN Book:MODERN PUBLICATIONChapter:ORGANIC COMPOUNDS CONTAINING NITROGEN Exercise:UNIT PRACTICE TEST
Explore 5 Videos
Similar Questions
Knowledge Check
Which of the following is not an alicyclic compound?
A(1)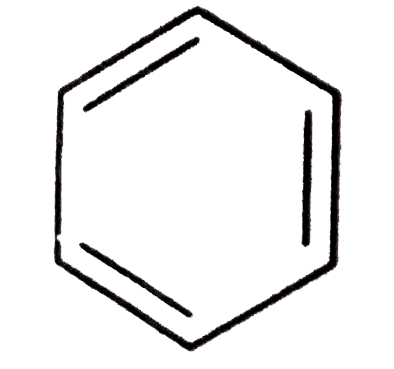 B(2)
B(2)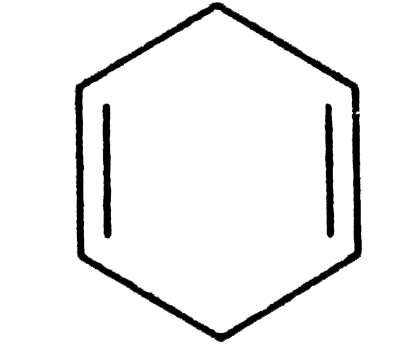 C(3)
C(3)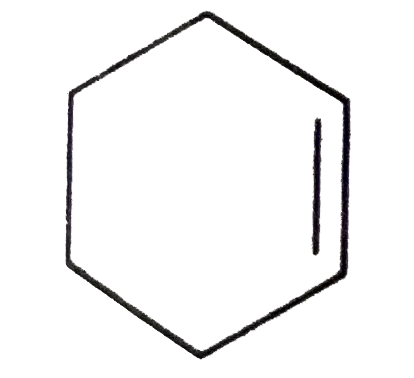 D(4)
D(4)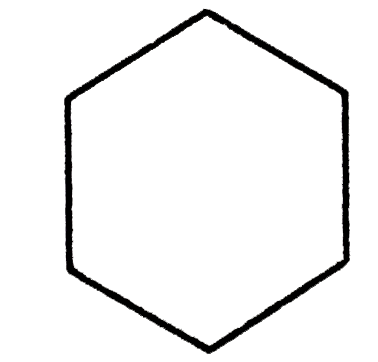
Which of the following is not a coordination compound ?
AK3[Fe(CN)6]BCoCl3.6NH3CK4[Fe(CN)]6DCarnalliteWhich of the following is not an aliphatic compound ?
A(1) Ethylene oxideB(2) IsobutyleneC(3) NeopentaneD(4) Acetaldehyde
