Alkylhalides have polar C-X bond and undergo nucleophilic substitution reactions. These give a variety of products with nucleophiles such as −OH,−OR,−NH2,−CN,−NC,−NO2,−ONO,RCOO−, etc. They undergo mainly two types of nucleophilic substitution reactions, SN1 and SN2. SN1 reactions are two steps reactions which proceed through the formation of carbocations while SN2 reactions are one step reaction which proceeds through the formation of transition state. The stability of carbocation and transition state determine the reactivity of alkyl halides.
Which out of SN1 or SN2 results into inversion of configuration?
To determine which mechanism, SN1 or SN2, results in inversion of configuration, we need to analyze the two types of nucleophilic substitution reactions.
1. Understanding Alkyl Halides: Alkyl halides have a polar C-X bond, where C is the carbon atom and X is the halogen. This polarity allows them to undergo nucleophilic substitution reactions.
2. Types of Nucleophilic Substitution Reactions: There are two main types of nucleophilic substitution reactions: SN1 and SN2.
3. Mechanism of S_(N)1 Reaction:
- The SN1 mechanism is a two-step process:
- Step 1: Formation of a carbocation by the departure of the leaving group (halogen).
- Step 2: Nucleophile attacks the carbocation.
- The nucleophile can attack from either side of the planar carbocation, leading to a racemic mixture of products (both enantiomers), which means there is no inversion of configuration.
4. Mechanism of S_(N)2 Reaction:
- The SN2 mechanism is a one-step process:
- The nucleophile attacks the carbon atom from the opposite side of the leaving group, leading to the formation of a transition state.
- As the nucleophile bonds to the carbon, the leaving group departs simultaneously.
- This back-side attack results in inversion of configuration at the carbon center, known as Walden inversion.
5. Conclusion: The SN2 mechanism results in inversion of configuration due to the back-side attack of the nucleophile, while the SN1 mechanism does not result in inversion due to the planar nature of the carbocation intermediate.
Final Answer:
The SN2 mechanism results in inversion of configuration.
---
- The SN1 mechanism is a two-step process:
- Step 1: Formation of a carbocation by the departure of the leaving group (halogen).
- Step 2: Nucleophile attacks the carbocation.
- The nucleophile can attack from either side of the planar carbocation, leading to a racemic mixture of products (both enantiomers), which means there is no inversion of configuration.
- The SN2 mechanism is a one-step process:
- The nucleophile attacks the carbon atom from the opposite side of the leaving group, leading to the formation of a transition state.
- As the nucleophile bonds to the carbon, the leaving group departs simultaneously.
- This back-side attack results in inversion of configuration at the carbon center, known as Walden inversion.
The SN2 mechanism results in inversion of configuration.
Topper's Solved these Questions
- HALOALKANES AND HALOARENESBOOK - MODERN PUBLICATIONCHAPTER - HALOALKANES AND HALOARENESEXERCISE - REVISION EXERCISE (Objective Questions) (Assertion Reason questions)
8 Videos
- HALOALKANES AND HALOARENESBOOK - MODERN PUBLICATIONCHAPTER - HALOALKANES AND HALOARENESEXERCISE - REVISION EXERCISE (Very short Answer Questions)
31 Videos
- HALOALKANES AND HALOARENESBOOK - MODERN PUBLICATIONCHAPTER - HALOALKANES AND HALOARENESEXERCISE - REVISION EXERCISE (Objective Questions) (Multiple choice questions)
30 Videos
- GENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTSBOOK - MODERN PUBLICATIONCHAPTER - GENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTSEXERCISE - COMPETION FILE (Integer Type Numerical Value Type Questions)
5 Videos
- ORGANIC COMPOUNDS CONTAINING NITROGEN BOOK - MODERN PUBLICATIONCHAPTER - ORGANIC COMPOUNDS CONTAINING NITROGEN EXERCISE - UNIT PRACTICE TEST
5 Videos
Similar Questions
Assertion: Addition of Br2 to 1-butane gives two optical isomers.
Reason: The product contains one asymmetric carbon atoms.
View Solutionhow will you prepare 1-bromopropane from propene?
View SolutionThe IUPAC name of (CH3)3CCl is
View SolutionAmongst following, total number of metals which occur in native state in the earth's crust are : Pt, Ni, C, Hg, S, Zn, Au, Pb
View SolutionConvert
(i) 3-Methylaniline into 3-nitrotoluene.
(ii) Aniline into 1,3,5 - tribromobenzene.
View SolutionAliphatic nucleophilic substitution is mainly of two type SN1 and SN2.SN2 reaction proceed with strong nucleophile in polar aprotic solvent. 3∘ halides do not give SN2 reaction. Inverted products are obtained in this reaction and mechanism of reaction occurs through the formation of transition state.
SN1 reaction proceed through the formation of carbocation in polar aprotic solvent. Solvent itself acts as nucleophile in this reaction. Racemization takes place in SN1 reaction.
Which of the following compounds will give SN1 reaction ?
View SolutionAliphatic nucleophilic substitution is mainly of two type SN1 and SN2.SN2 reaction proceed with strong nucleophile in polar aprotic solvent. 3∘ halides do not give SN2 reaction. Inverted products are obtained in this reaction and mechanism of reaction occurs through the formation of transition state.
SN1 reaction proceed through the formation of carbocation in polar aprotic solvent. Solvent itself acts as nucleophile in this reaction. Racemization takes place in SN1 reaction.
Which one of the following will give racemised product in C2H5OH?
View SolutionAliphatic nucleophilic substitution is mainly of two type SN1 and SN2.SN2 reaction proceed with strong nucleophile in polar aprotic solvent. 3∘ halides do not give SN2 reaction. Inverted products are obtained in this reaction and mechanism of reaction occurs through the formation of transition state.
SN1 reaction proceed through the formation of carbocation in polar aprotic solvent. Solvent itself acts as nucleophile in this reaction. Racemization takes place in SN1 reaction.
Which one of the following will give SN2 reaction?
View SolutionAlkylhalides have polar C-X bond and undergo nucleophilic substitution reactions. These give a variety of products with nucleophiles such as -OH, -OR, -NH_(2), -CN, -NC, -NO_(2), -ONO, RCOO^(-) , etc. They undergo mainly two types of nucleophilic substitution reactions, S_(N)1 and S_(N)2 . S_(N)1 reactions are two steps reactions which proceed through the formation of carbocations while S_(N)2 reactions are one step reaction which proceeds through the formation of transition state. The stability of carbocation and transition state determine the reactivity of alkyl halides. Write the main products A and B in the following reaction. A overset(KNO_(2))larr CH_(3)CH_(2)Br overset(AgNO_(2))to B .
View SolutionAlkylhalides have polar C-X bond and undergo nucleophilic substitution reactions. These give a variety of products with nucleophiles such as −OH,−OR,−NH2,−CN,−NC,−NO2,−ONO,RCOO−, etc. They undergo mainly two types of nucleophilic substitution reactions, SN1 and SN2. SN1 reactions are two steps reactions which proceed through the formation of carbocations while SN2 reactions are one step reaction which proceeds through the formation of transition state. The stability of carbocation and transition state determine the reactivity of alkyl halides.
Complete the reaction:
CH3CH2BrAg2O−−→Heat
View SolutionAlkylhalides have polar C-X bond and undergo nucleophilic substitution reactions. These give a variety of products with nucleophiles such as −OH,−OR,−NH2,−CN,−NC,−NO2,−ONO,RCOO−, etc. They undergo mainly two types of nucleophilic substitution reactions, SN1 and SN2. SN1 reactions are two steps reactions which proceed through the formation of carbocations while SN2 reactions are one step reaction which proceeds through the formation of transition state. The stability of carbocation and transition state determine the reactivity of alkyl halides.
Arrange the following in the increasing order of reactivity towards SN2 reaction:
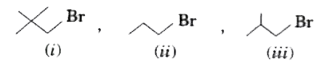
View SolutionAlkylhalides have polar C-X bond and undergo nucleophilic substitution reactions. These give a variety of products with nucleophiles such as -OH, -OR, -NH_(2), -CN, -NC, -NO_(2), -ONO, RCOO^(-) , etc. They undergo mainly two types of nucleophilic substitution reactions, S_(N)1 and S_(N)2 . S_(N)1 reactions are two steps reactions which proceed through the formation of carbocations while S_(N)2 reactions are one step reaction which proceeds through the formation of transition state. The stability of carbocation and transition state determine the reactivity of alkyl halides. Write the structure of isomer of compound C_(4)H_(9)Br which is most reactive towards S_(N)1 reaction.
View SolutionAlkylhalides have polar C-X bond and undergo nucleophilic substitution reactions. These give a variety of products with nucleophiles such as -OH, -OR, -NH_(2), -CN, -NC, -NO_(2), -ONO, RCOO^(-) , etc. They undergo mainly two types of nucleophilic substitution reactions, S_(N)1 and S_(N)2 . S_(N)1 reactions are two steps reactions which proceed through the formation of carbocations while S_(N)2 reactions are one step reaction which proceeds through the formation of transition state. The stability of carbocation and transition state determine the reactivity of alkyl halides. Which out of S_(N)1 or S_(N)2 results into inversion of configuration?
View SolutionAlkyl halides undergo nucleophilic substitution reactions in which halogen atom is replaced by other atom.
RX+Nu:−→Nu−R+X−
These reactions follow SN1 and SN2 type mechanism, in which SN1 takes place in two steps while SN2 takes place in single step. Due to their tendency to undergo substitution by a large number of nucleophiles, they form a variety of products.
Which of the following is least reactive towards SN2 mechanism ?
View SolutionAlkyl halides undergo nucleophilic substitution reactions in which halogen atom is replaced by other atom.
RX+Nu:−→Nu−R+X−
These reactions follow SN1 and SN2 type mechanism, in which SN1 takes place in two steps while SN2 takes place in single step. Due to their tendency to undergo substitution by a large number of nucleophiles, they form a variety of products.
In which of the following nucleophilic substitution reaction, the product formed is racemic mixture ?
View Solution

