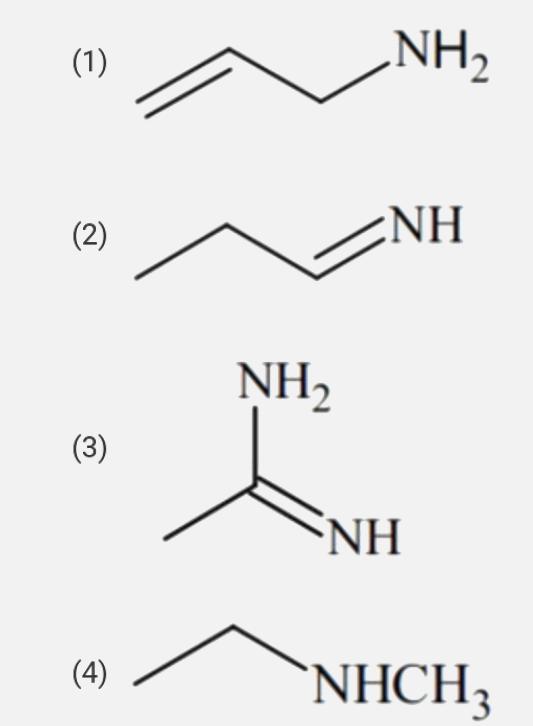
The order of basicity among the following compounds is

A
IV>II>III>I
B
II>I>IV>III
C
I>IV>III>II
D
IV>I>II>III
Text Solution
The correct Answer is:D
 Resonance with two NH2 groups increases electron density on N of =NH.
Resonance with two NH2 groups increases electron density on N of =NH.
 Resonance with one NH2 group increases electron density on N to lesser extent (than IV) of = NH.
Resonance with one NH2 group increases electron density on N to lesser extent (than IV) of = NH.
 Electron density on N of NH increases due to resonance
Electron density on N of NH increases due to resonance
 The lone pair is not available because it is involved in aromatic sextet.
The lone pair is not available because it is involved in aromatic sextet.
Hence, correct order is IV>I>II>III
|
Similar Questions
Knowledge Check
The order of basicity among the the following compounds is
 AII>I>IV>IIIBI>IV>III>ICIV>I>II>IDIV>I>II>III
AII>I>IV>IIIBI>IV>III>ICIV>I>II>IDIV>I>II>IIIThe order of basicity of the following compounds is
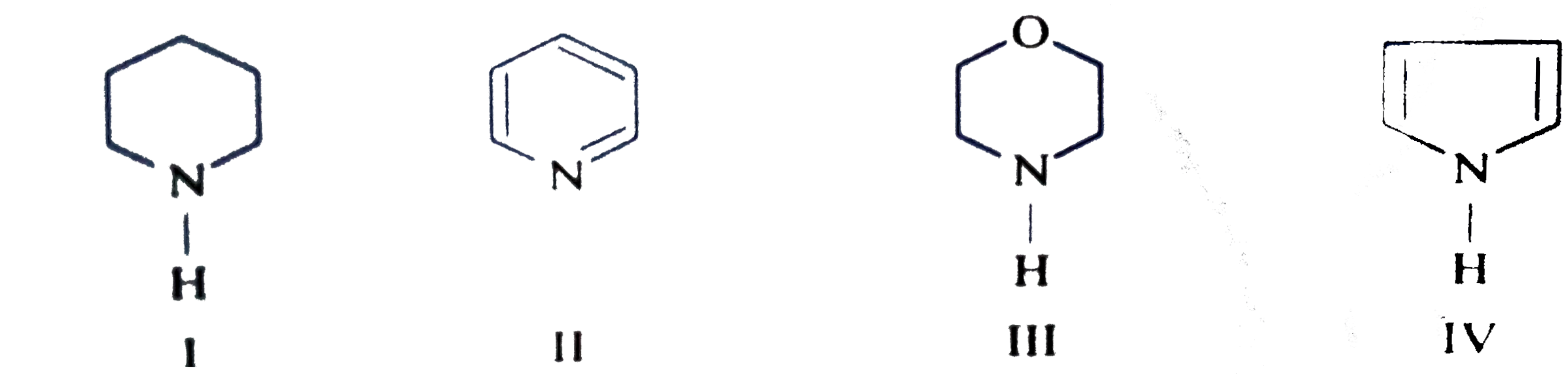
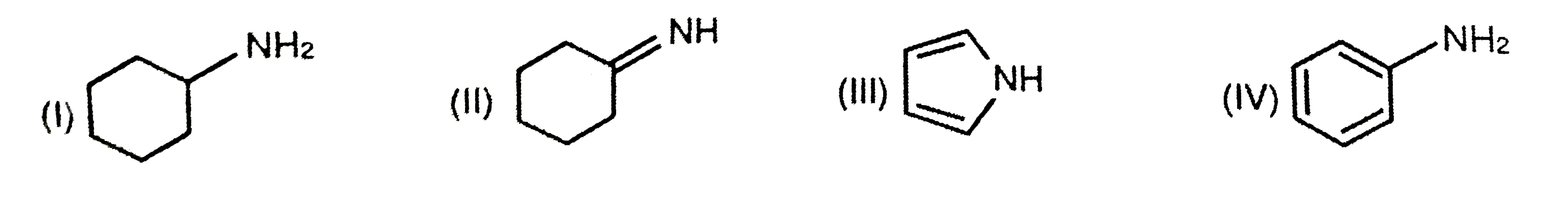 AI>II>IV>IIIBIV>II>I>IICIII>II>I>IVDI>II>III>IV
AI>II>IV>IIIBIV>II>I>IICIII>II>I>IVDI>II>III>IVThe order of basicity of the following compounds is
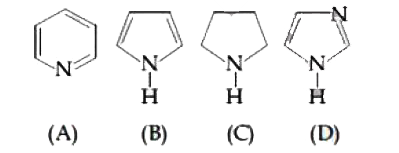
 AIV>I>III>IIBIII>I>IV>IICII>I>III>IVDI<III<II<IV
AIV>I>III>IIBIII>I>IV>IICII>I>III>IVDI<III<II<IV
The increasing order of the basicity of the following compounds is :
View Solution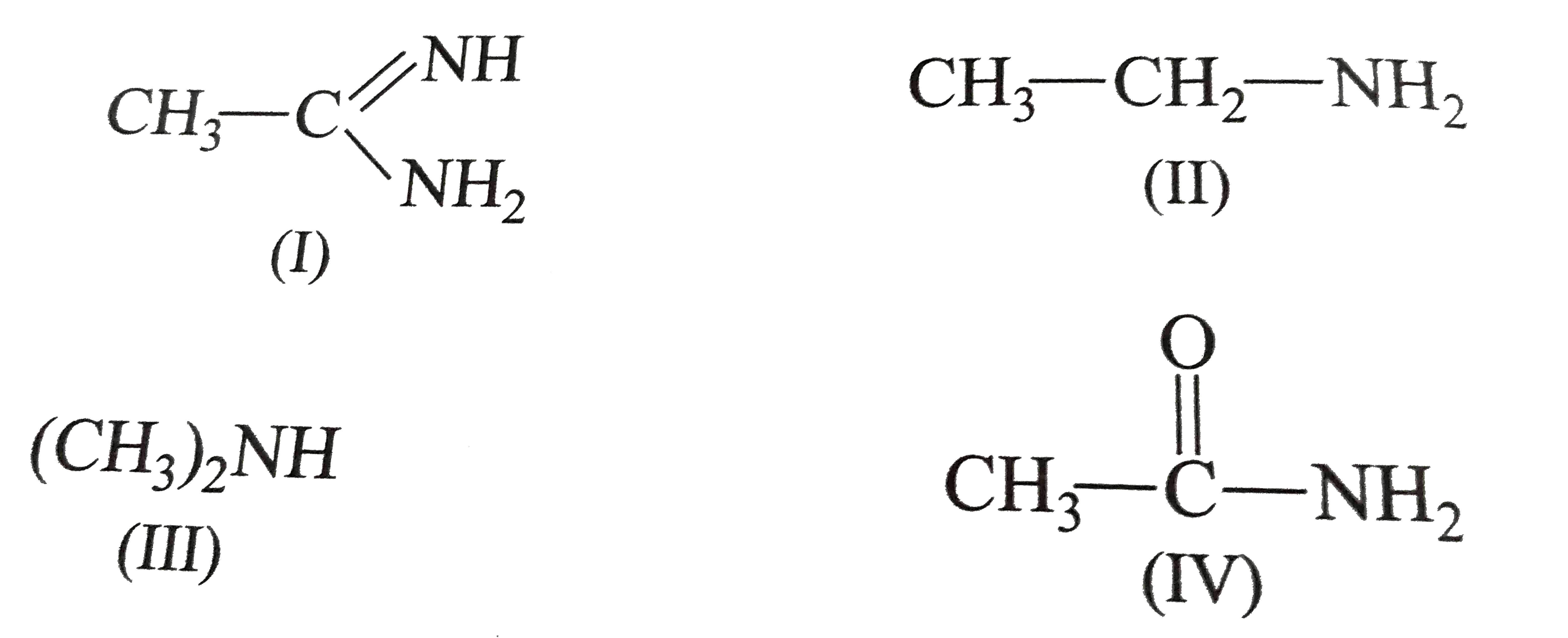
What is the correct order of basicity among the following compounds?
View SolutionThe correct order of basicity of the following compounds is

View SolutionThe increasing order of basicity of the following compounds is
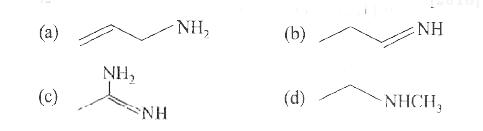
View SolutionThe increasing order of basicity of the following compounds is :

View Solution