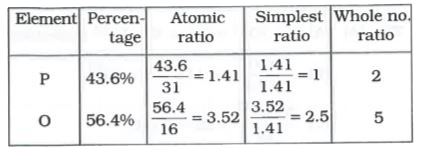
A phosphorus oxide has 43.6% phosphorus (at. mass=31). The empirical formula of the compound is :Solution in Kannada
A
P2O5
B
P2O3
C
P4O6
D
PO2
Video Solution
Text Solution
The correct Answer is:A
Formula P2O5
|
Similar Questions
Calculate the molar mass of Phosphorus molecule , P_(4) (Atomic mass of phosphorus = 31)
View Solutionफांस्फोरस हाइड्राइड यौगिक के सूत्र लिखिए ?
View SolutionA phosphorus oxide has 43.6% phosphorus (at. mass = 31). The empirical formula of the compound is
View SolutionAssertion : Oxidation number of phosphorus in P_4 is zero. Reason : Phosphorus has oxidation state zero in all its compound .
View SolutionCalculate the molar mass of the following substances :Phosphorus molecule,P4 (Atomic mass of phosphorus is 31)
View SolutionWhat is the formula, name and molecular mass of compound. Which obtain by estimation of phosphorus?
View SolutionWhat is the formula, name and molecular mass of compound. Which obtain by estimation of phosphorus?
View Solution
