প্রদত্ত রেডক্স বিক্রিয়াটি একটি গ্যালভানীয় কোশে সংঘটিত হয়: Zn(s)+Cu2+(0.1M)→Zn2+(1M)+Cu(s)।যদি E0Zn2+∣Zn = -0.76V E0Cu2+∣Cu = +0.34V এবং হয় তবে 25°C উষ্ণতায় E_(cell)-এর মান-
1.04V
1.10V
1.16V
1.07V
Step by step video & image solution for প্রদত্ত রেডক্স বিক্রিয়াটি একটি গ্যালভানীয় কোশে সংঘটিত হয়: Zn(s)+Cu^(2+) (0.1M)rarrZn^(2+)(1M)+Cu(s)।যদি E_(Zn^(2+)|Zn]^0 = -0.76V E_(Cu^(2+)|Cu]^0 = +0.34V এবং হয় তবে 25°C উষ্ণতায় E_(cell)-এর মান- by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
Determine true statement for Zn-Cu electrochemical cell. [E_(Zn^(+2)|Zn)^(@)=-0.76V,E_(Cu|Cu^(+2))^(@)=-0.34V]
View SolutionCalculate the e.m.f. of the cell
Zn/Zn2+(0.1M)||Cu2+(0.01M)|Cu
EΘZn2+/Zn=−0.76 V and EΘCu2+/Cu=0.34V
View SolutionCalculate Ecell at 25∘C for the reaction :
Zn+Cu2+(0.20M)→Zn2+(0.50M)+Cu
Given EΘ(Zn2+/Zn)=−0.76V,EΘ(Cu2+/Cu)=0.34V.
View SolutionFind out the E_("cell")^(@) from the given data (a) Zn|Zn^(+2)|| Cu^(+2)| Cu, E_("cell")^(@) = 1.10V (b) Cu| Cu^(+2)|| Ag^(+)|Ag, E_("cell")^(@) = 0.46V ( c) Zn|Zn^(+2)||Ag^(+) | Ag, E_("cell")^(@) = ? (Given E_(Cu^(+2)//Cu)^(@) = 0.34V )
View SolutionFor the given cell arrangement identify incorrect statement
given E∘_(Cu2+/Cu) = 0.34V &E∘_(Zn2+/Zn)=−0.76V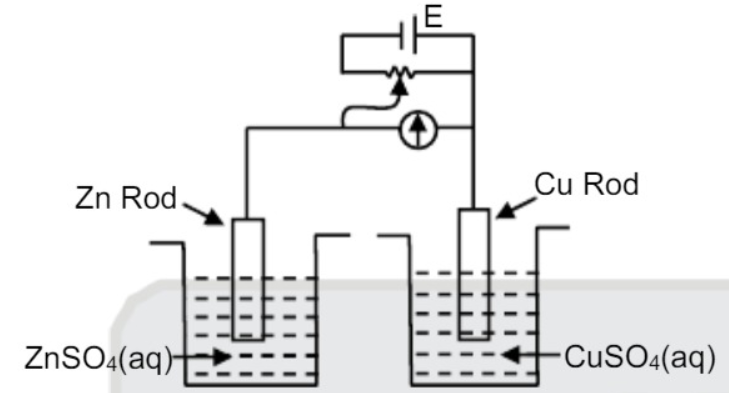
View Solutionअभिक्रिया का E∘सेल है
Zn(s)+Cu+2(aq)→Zn+2(aq)+Cu(s)
[यदि E∘Zn2+/Zn=0.76 वोल्ट, E∘Cu2+/Cu=0.34 वोल्ट]
View SolutionCalculate the standard Gibbs energy for the cell : Zn(s)+Zn^(2+)(aq)||Cu^(2+)(aq)|Cu(s) E_((Zn^(2+)//Zn)^(@))=-0.76V,E_((Cu^(2+)//Cu))^(@)=0.34V , F=96500 C .
View SolutionCalculate the equilibrium constant for the reaction at 298K. Zn(s) +Cu^(2+)(aq) hArr Zn^(2+)(aq) +Cu(s) Given, E_(Zn^(2+)//Zn)^(@) =- 0.76V and E_(Cu^(2+)//Cu)^(@) = +0.34 V
View Solution

