Define 'binding energy'. Sketch the graph between binding energy per nucleon and mass number.
Step-by-Step Solution:
1. Definition of Binding Energy:
- Binding energy is defined as the energy required to hold the nucleons (protons and neutrons) together in the nucleus of an atom. It represents the stability of the nucleus; a higher binding energy indicates a more stable nucleus.
2. Understanding Nucleons:
- Nucleons are the particles found in the nucleus of an atom. They include protons (positively charged) and neutrons (neutral). The binding energy is the energy needed to overcome the forces that hold these nucleons together.
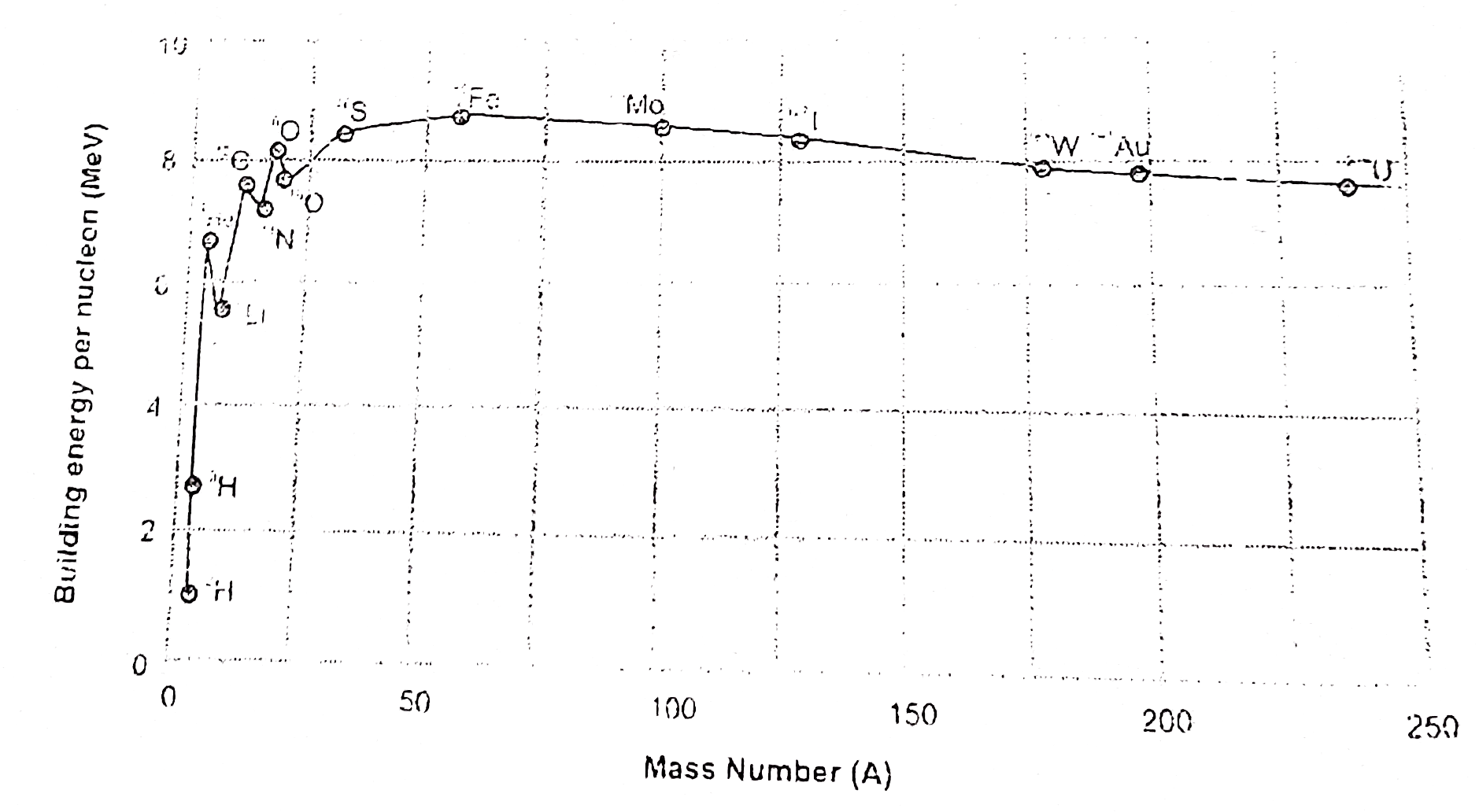
3. Graphing Binding Energy per Nucleon vs. Mass Number:
- To sketch the graph, we will plot binding energy per nucleon (on the y-axis) against mass number (on the x-axis).
- The mass number (A) represents the total number of protons and neutrons in the nucleus.
4. Key Points for the Graph:
- Start by marking the mass numbers on the x-axis. Common mass numbers to consider include:
- Hydrogen (A=1)
- Helium (A=4)
- Lithium (A=7)
- Beryllium (A=9)
- Carbon (A=12)
- Nitrogen (A=14)
- Oxygen (A=16)
- Iron (A=56)
- Lead (A=207)
- On the y-axis, mark the binding energy per nucleon values. Typical values are:
- Hydrogen: ~1 MeV
- Helium: ~7 MeV
- Carbon: ~7.5 MeV
- Iron: ~8.8 MeV
- Lead: ~7.2 MeV
5. Sketching the Graph:
- Begin plotting the points based on the mass number and corresponding binding energy per nucleon values.
- The graph typically shows a curve that rises to a peak around iron (A=56) and then starts to decline for heavier elements like lead.
6. Interpreting the Graph:
- The peak indicates that iron has one of the highest binding energies per nucleon, suggesting it is one of the most stable nuclei.
- The decline in binding energy per nucleon for heavier elements indicates that they are less stable and more prone to nuclear reactions.
- Binding energy is defined as the energy required to hold the nucleons (protons and neutrons) together in the nucleus of an atom. It represents the stability of the nucleus; a higher binding energy indicates a more stable nucleus.
- Nucleons are the particles found in the nucleus of an atom. They include protons (positively charged) and neutrons (neutral). The binding energy is the energy needed to overcome the forces that hold these nucleons together.
- To sketch the graph, we will plot binding energy per nucleon (on the y-axis) against mass number (on the x-axis).
- The mass number (A) represents the total number of protons and neutrons in the nucleus.
- Start by marking the mass numbers on the x-axis. Common mass numbers to consider include:
- Hydrogen (A=1)
- Helium (A=4)
- Lithium (A=7)
- Beryllium (A=9)
- Carbon (A=12)
- Nitrogen (A=14)
- Oxygen (A=16)
- Iron (A=56)
- Lead (A=207)
- On the y-axis, mark the binding energy per nucleon values. Typical values are:
- Hydrogen: ~1 MeV
- Helium: ~7 MeV
- Carbon: ~7.5 MeV
- Iron: ~8.8 MeV
- Lead: ~7.2 MeV
- Begin plotting the points based on the mass number and corresponding binding energy per nucleon values.
- The graph typically shows a curve that rises to a peak around iron (A=56) and then starts to decline for heavier elements like lead.
- The peak indicates that iron has one of the highest binding energies per nucleon, suggesting it is one of the most stable nuclei.
- The decline in binding energy per nucleon for heavier elements indicates that they are less stable and more prone to nuclear reactions.
Similar Questions
Variation Of Binding Energy Of Per Nucleon With Mass Number
View SolutionThe graph between the binding energy per nucleon (E) and atomic mass number (A) is as-
View SolutionDraw the graph showing thervariation of binding energy per nucleon with mass number. Give therason for the decrease of binding energy per nucleon for nuclei with high mass numbers. The binding energy per nucleon for nuclei with high mass number is low due to the large coulomb repulsion between the protons inside these nuclei.
View SolutionBinding energy per nucleon relation with mass number
View SolutionAs the mass number increase binding energy per nucleon :
View SolutionWhat is Mass defect, binding energy and binding energy per nucleon?
View SolutionDefine Binding energy of the nucleus. Draw and explain curve between Binding Energy per nucleon and mass number.
View SolutionDefine binding energy, binding energy per nucleon. Draw and explain a curve between binding energy per nucleon and mass number.
View SolutionDefine :Binding energy per nucleon. Express binding energy per nucleon in terms of mass defect.
View SolutionBinding energy curve shows the variation of Binding energy per nucleon of nuclei with mass number. Binding energy per nucleon is maximum for mass number..
View SolutionDraw the graph showing thervariation of binding energy per nucleon with mass number. Give therason for the decrease of binding energy per nucleon for nuclei with high mass numbers.
The binding energy per nucleon for nuclei with high mass number is low due to the large coulomb repulsion between the protons inside these nuclei.
View SolutionThe graph between the binding energy per nucleon (E) and atomic mass number (A) is as-
View SolutionDraw the graph showing thervariation of binding energy per nucleon with mass number. Give therason for the decrease of binding energy per nucleon for nuclei with high mass numbers.

The binding energy per nucleon for nuclei with high mass number is low due to the large coulomb repulsion between the protons inside these nuclei.
View SolutionExplain Binding energy and binding energy per nucleon.
View SolutionDefine binding energy per nucleon. Draw the curve between mass number and average binding energy.
View Solution

