Explain the following :
(a) PH3 has lower boiling point than NH3 , why
(b) Noble gases are mostly inert why ?
(a) This is because in ammonia molecules there are intermolecular H-bond but in PH3 there are weak vander waal.s forces of attraction .
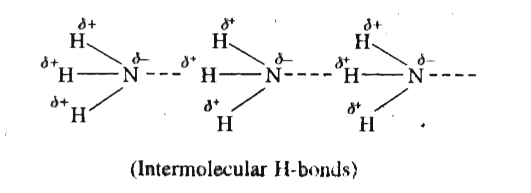
(b) This is due to the following reasons :
(i) They have high ionization enthalpies. (ii) They have zero election affinity. (iii) They have their octets complete. (iv) They have no unpaired electrons.
Similar Questions
PH3 has lower boiling point that NH3. Why ?
View SolutionPH3 has lower boiling point that NH3. Why ?
View SolutionWhy does PH3 have lower boiling point than NH3 ?
View SolutionGive reasons for the following:
NH3 has a higher boiling point than PH3.
View SolutionGive reasons for the following:
NH3 has higher boiling point than PH3.
View Solution(a) Given reasons for the following :
(i) Bond enthalpy of F2 is lower than that of Cl2.
(ii) PH3 has lower boiling point than NH3.
(b) Draw the structures of the following molecules :
(i) BrF3 (ii) (HPO3)3
(iii) XeF4
View Solution(a) Given reasons for the following :
(i) Bond enthalpy of F2 is lower than that of Cl2.
(ii) PH3 has lower boiling point than NH3.
(b) Draw the structures of the following molecules :
(i) BrF3 (ii) (HPO3)3
(iii) XeF4
View SolutionWhy does methylamine has lower boiling point than methanol ?
View SolutionWhy do noble gases have low boiling points ?
View SolutionWhy do noble gases have low boiling points ?
View Solution

