Why are alcohols weaker acids than water?
This because in alcohols, due to +I− effect of alkyl group, the electron around O− atom increases and loss of H− atom as proton becomes difficult as compared to in water.
R→O−H⇔R−O−+H+H−O−H⇔HO−+H+
Similar Questions
Knowledge Check
Assertion : Methyl alcohol is a weaker acid than water.
Reason : Among the aliphatic monohydric alcohols, methyl alcohol is the strongest acid.AIf both assertion and reason are correct and reason is correct explanation for assertion.BIf both assertion and reason are correct but reason is not correct explanation for assertion.CIf assertion is correct but reason is incorrect.DIf assertion and reason both are incorrect.Select the correct statement(s).
I. Alcohols are weaker acid than water
II. Water is a better proton donor than alcohol
III. Sodium ethoxide is a stronger base than sodium hydroxideAOnly IBII and IIICOnly IIIDAll of theseAlcohols are acidic in nature because hydrogen is attached to oxygen which is an electronegative element. Alcohol is weaker acid than water because OH− ion is more stable than R−O− ion. R−O− is less stable, due to +I effect of the alkyl group. Acidity of alcohols increases with increase in the stability of acid anion.
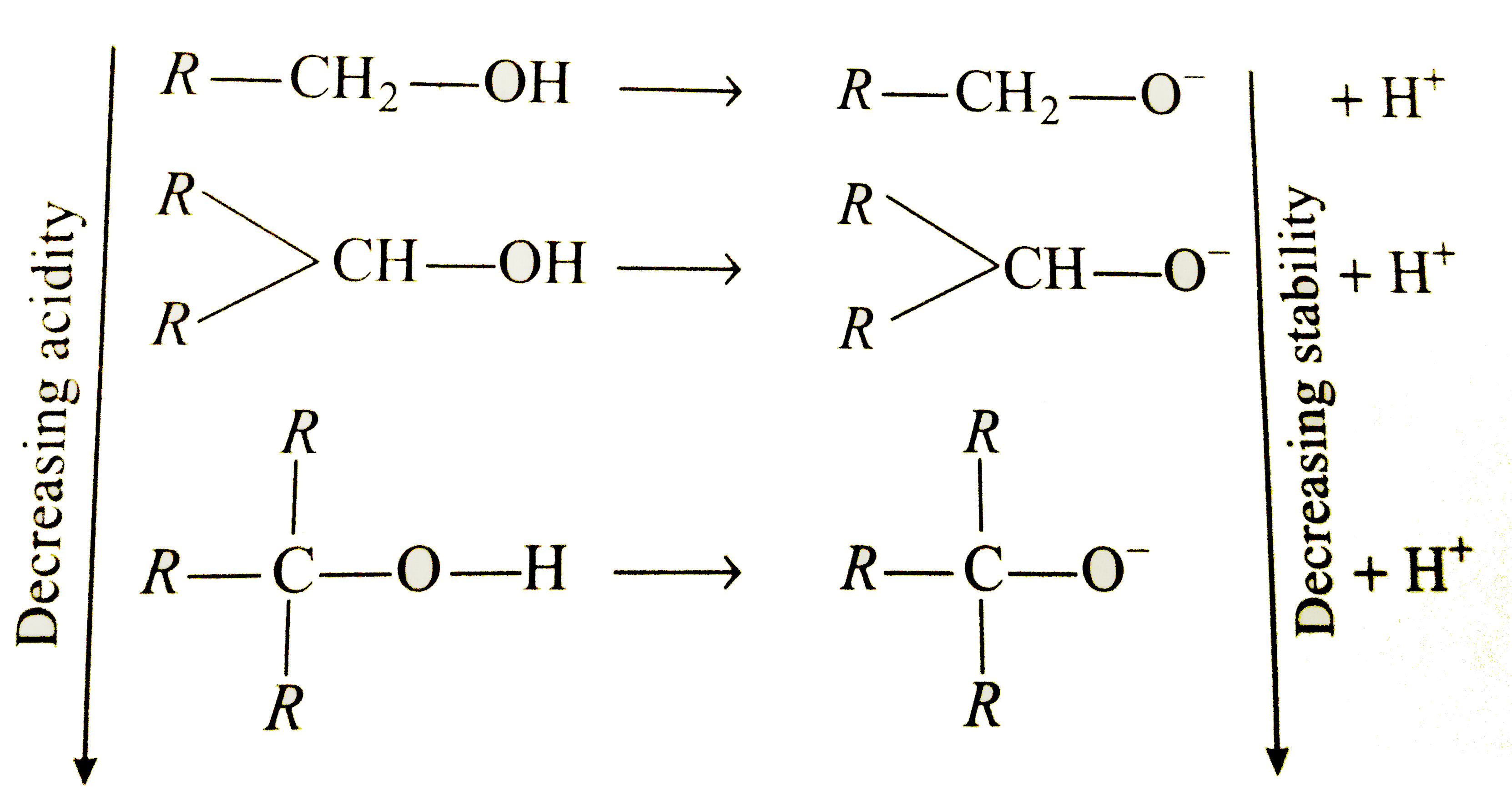
Some alcohol like CH3−OH,CF3−CH2−OH,(CF3)3C−OH ae more acidic than water.
Which among the following alcohols has highest pKa value ?ACH3−CH2−CH2−CH2−OHBCH3−CH3∣C−CH2−CH3CCH3−OH∣CH−CH2−CH3DCH3−CH3∣C∣CH3−OH
Why is ethanoic acid weaker acid than benzoic acid ?
View SolutionAlcohols are acidic in nature because hydrogen is attached to oxygen which is an electronegative element. Alcohol is weaker acid than water because OH− ion is more stable than R−O− ion. R−O− is less stable, due to +I effect of the alkyl group. Acidity of alcohols increases with increase in the stability of acid anion.
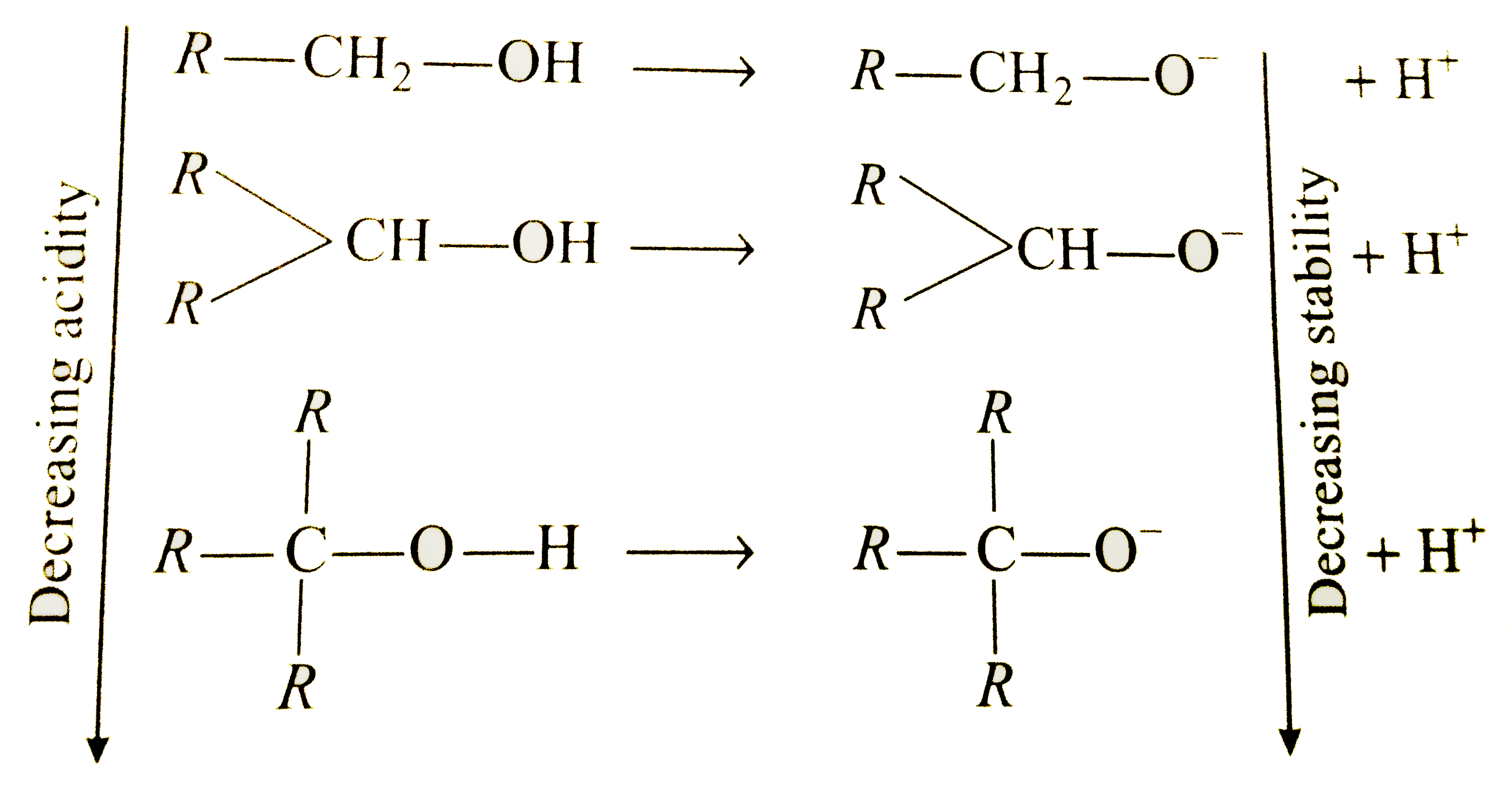
Some alcohol like CH3−OH,CF3−CH2−OH,(CF3)3C−OH ae more acidic than water.
Which among the following is the most acidic ?
View SolutionAlcohols are acidic in nature because hydrogen is attached to oxygen which is an electronegative element. Alcohol is weaker acid than water because OH− ion is more stable than R−O− ion. R−O− is less stable, due to +I effect of the alkyl group. Acidity of alcohols increases with increase in the stability of acid anion.
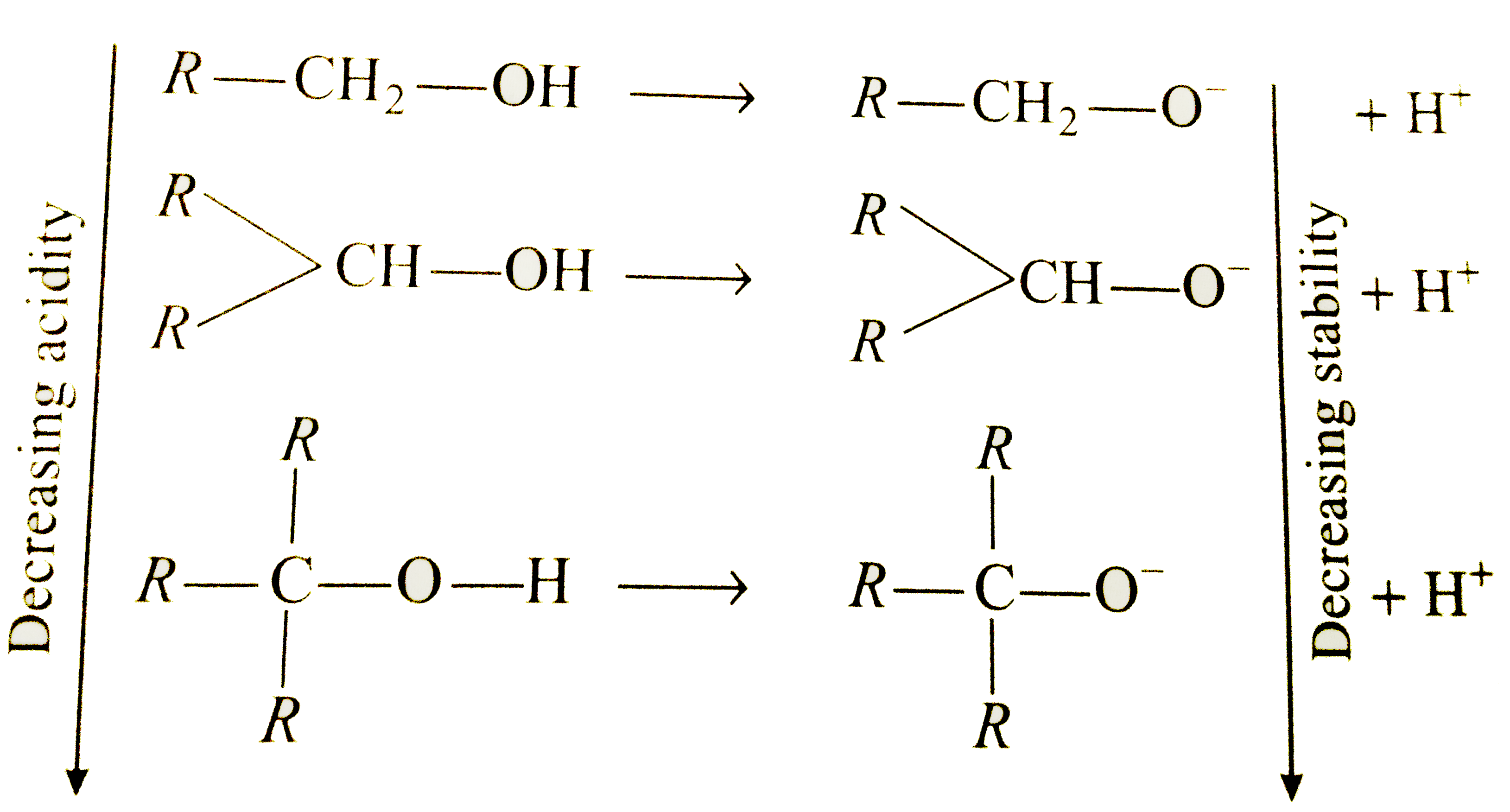
Some alcohol like CH3−OH,CF3−CH2−OH,(CF3)3C−OH ae more acidic than water.
Which of the following will react fastest with sodium ?
View SolutionAlcohols are acidic in nature because hydrogen is attached to oxygen which is an electronegative element. Alcohol is weaker acid than water because OH− ion is more stable than R−O− ion. R−O− is less stable, due to +I effect of the alkyl group. Acidity of alcohols increases with increase in the stability of acid anion.
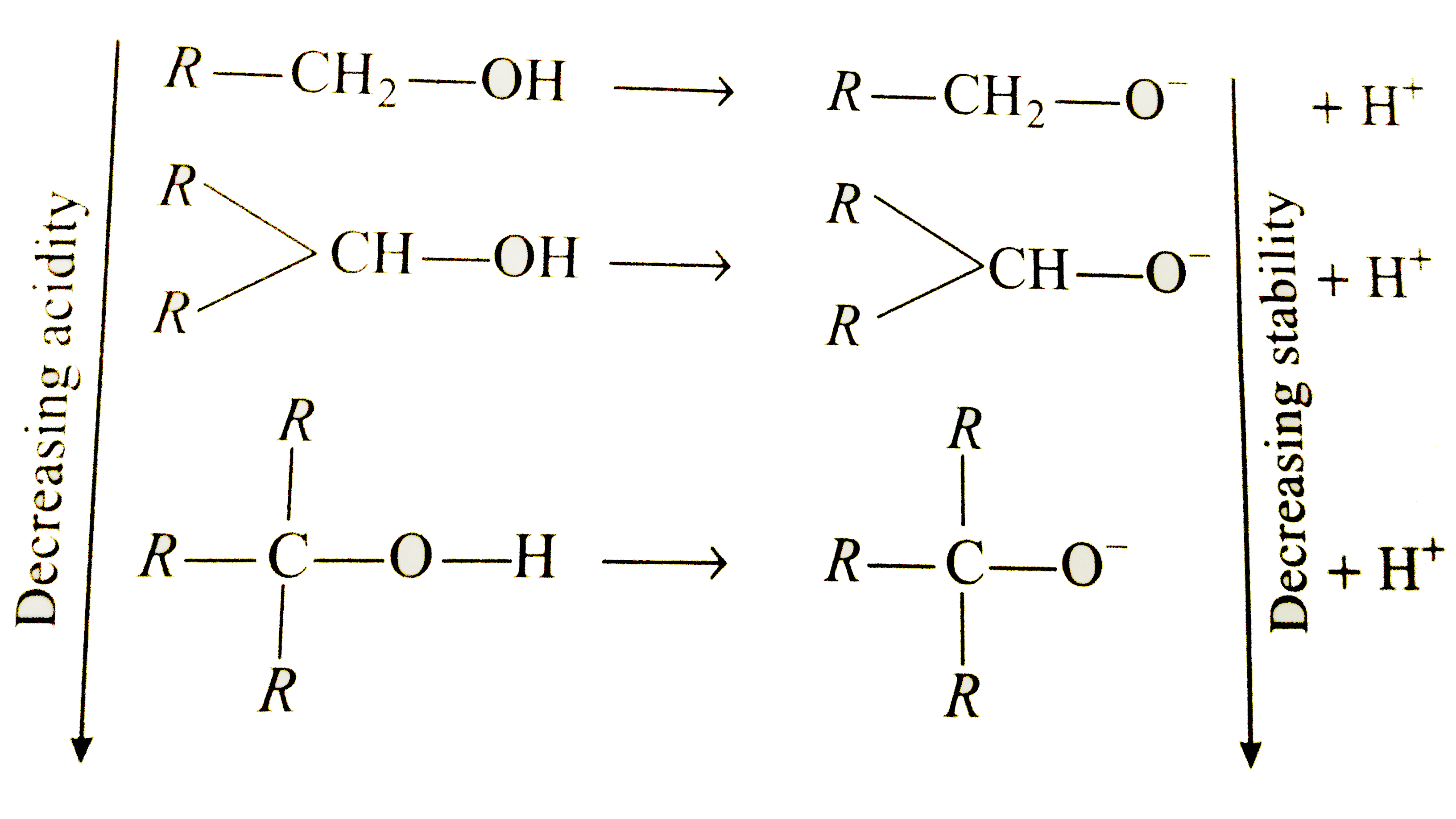
Some alcohol like CH3−OH,CF3−CH2−OH,(CF3)3C−OH ae more acidic than water.
The strongest conjugate base out of the following is :
View SolutionAsseriton: Methanol is stronger acid than water.
Reason: All alcohols are stronger acod than water.
View Solution

