The Van der Waals constants for two gases A and B are as follows :
Gasa(atm L2 mol−2)b(L mol−1) A 1.63 0.0326 B 3,72 0.0521
Which of these
(i) is more easily liquefied?
(ii) has larger molecular size?
(i) Greater the value of .a. more easily the gas is liquefied. Hence gas B is more easily liquefied.
(ii) Larger the value of .b., larger is is its molecular size. Therefore, B has larger molecular size.
Topper's Solved these Questions
Similar Questions
The van der Waals constants for two gases are as follows :
Gasa(atmL2mol−2)b(LMol−1)X1.390.0391Y3.590.0427
Which of them is more easily liquefiable and which has greater molecular size ?
View SolutionThe van der Waal's constants for two gases are as follows:
Gas a (atm L2 mol−2) b(Lmol−1)
X 1.39 0.0391
Y 3.59 0.0427
Which of them is more easily liqufiable and which has greater molecular size ?
View Solution
Knowledge Check
van der Waal's constants of two gases X and Y are as given:
a(litre - atom mol−2)b(litre mol−1)Gas X 5.6 0.065Gas Y 5.1 0.012
What is correct about the two gases ?ATc(X)>Tc(Y)BTc(X)=Tc(Y)CVc(X)>Vc(Y)DVc(Y)>Vc(X)If the Vander Waal's constant of gas A are given as -
a ( atm L2mol2) =6.5
b( L mol1 ) 0.056
than critical pressure of A isA56.24B76.77 atmC42.44atmD36.42 atmvan der Waal's constants for gases A,B and C are as follows:
Gas a(kPa dm6mol−2)B(L/mol)A4100.027B12150.03C6080.032
Which of the following options contain correct arrangement of "gas with highest critical temperature" ad "gas with most ideal behaviour around 1850K" respectively?AB,ABA,BCC,BDB,C
The Van der Waals constants of gas A are given as:
a ( atm L2 mol−2)=8,6(Lmol−1)=0.060
The critical pressure of A is ------ atm.
View SolutionThe van der Waal constants of two gases are as follows :
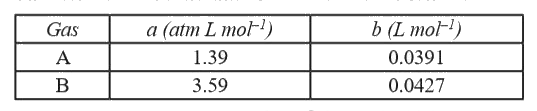
Which of them is more easily liquefiable and which has greater molecular size?
View SolutionThe vander Waals constants for gases A,B and C are as follows
Gas a/dm6kPamol−2 b/dm3mol−1
A 405.3 0.027
B 1215.9 0.030
C 607.95 0.032
Which gas has the largest molecular volume.
View SolutionThe vander Waals constants for gases A,B and C are as follows
Gas a/dm6kPamol−2 b/dm3mol−1
A 405.3 0.027
B 1215.9 0.030
C 607.95 0.032
Which gas has the highest critical temperature.
View SolutionThe van der Waals constant for gases A, B, and C are as follows

Answer the following:
Which gas has the largest molecular volume?
View Solution
