One mole of an ideal monoatomic gas is taken through a polytropic process P2T = constant. The heat required to increase the temperature of the gas by ΔT is
RΔT
3RΔT
5RΔT
6RΔT
The correct Answer is:B
Topper's Solved these Questions
- PHYSICS PART2BOOK - FIITJEECHAPTER - PHYSICS PART2EXERCISE - Section (I) : Subjective
4 Videos
- PHYSICS PART2BOOK - FIITJEECHAPTER - PHYSICS PART2EXERCISE - Section (II) : MCQ
27 Videos
- PHYSICS PART-IIIBOOK - FIITJEECHAPTER - PHYSICS PART-IIIEXERCISE - NUMERICAL BASED QUESTIONS DECIMAL TYPE
11 Videos
- ROTATIONAL MECHANICSBOOK - FIITJEECHAPTER - ROTATIONAL MECHANICSEXERCISE - COMPREHENSION - IV
2 Videos
Similar Questions
Knowledge Check
One mole of an ideal monoatomic gas is taken through a cyclic process as shown. Choose the correct option(s).
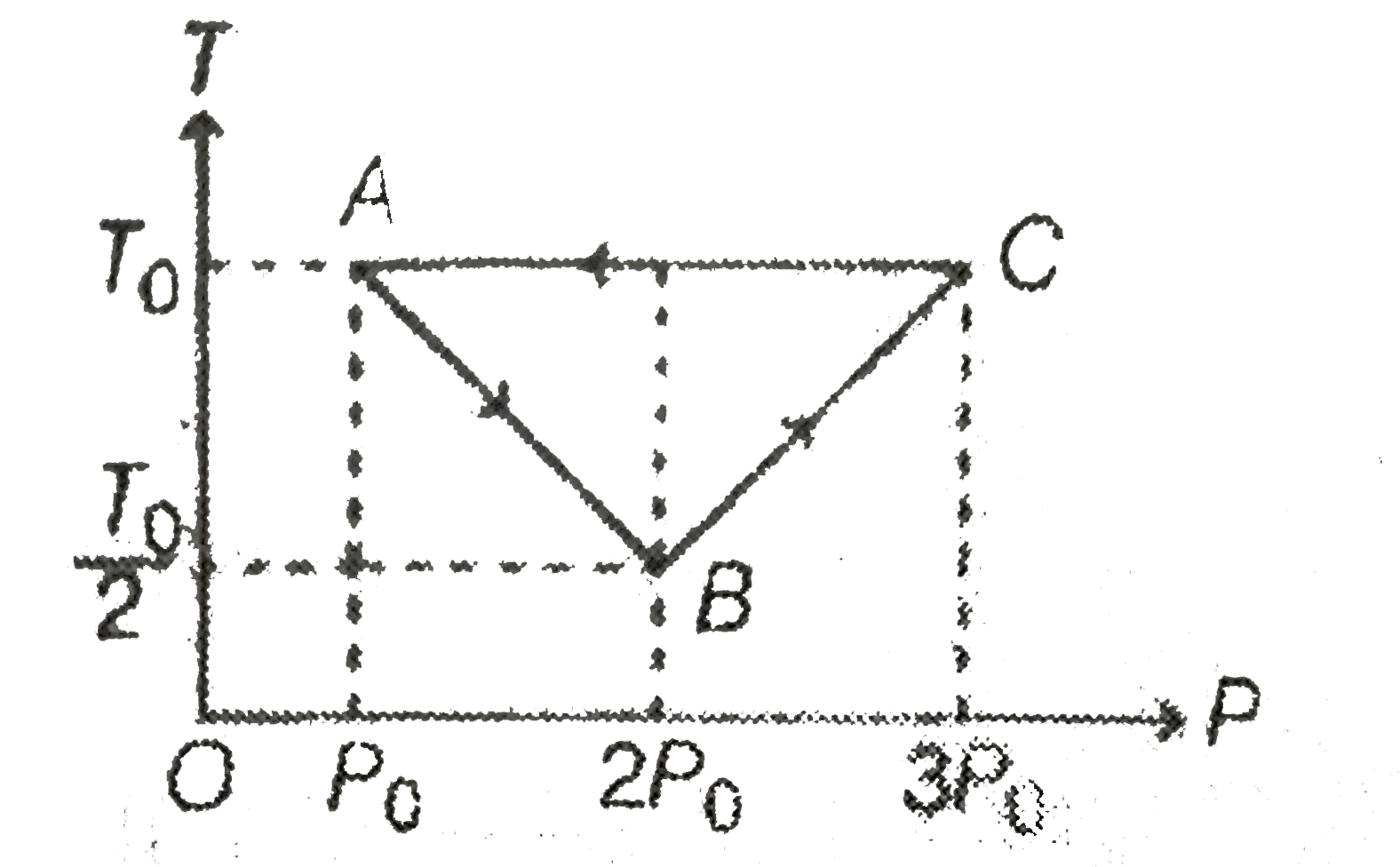 AWork done in process CA is RT0ln3.BHeat given to the gas in process CA is RT0ln3CThe magnitude of change in internal energy in process AB and BC must be sameDThe change in internal energy in process AB is negative.
AWork done in process CA is RT0ln3.BHeat given to the gas in process CA is RT0ln3CThe magnitude of change in internal energy in process AB and BC must be sameDThe change in internal energy in process AB is negative.One mole of an ideal monoatomic gas is taken through the thermodynamic process shown in the P−V diagram. The heat supplied to the system is
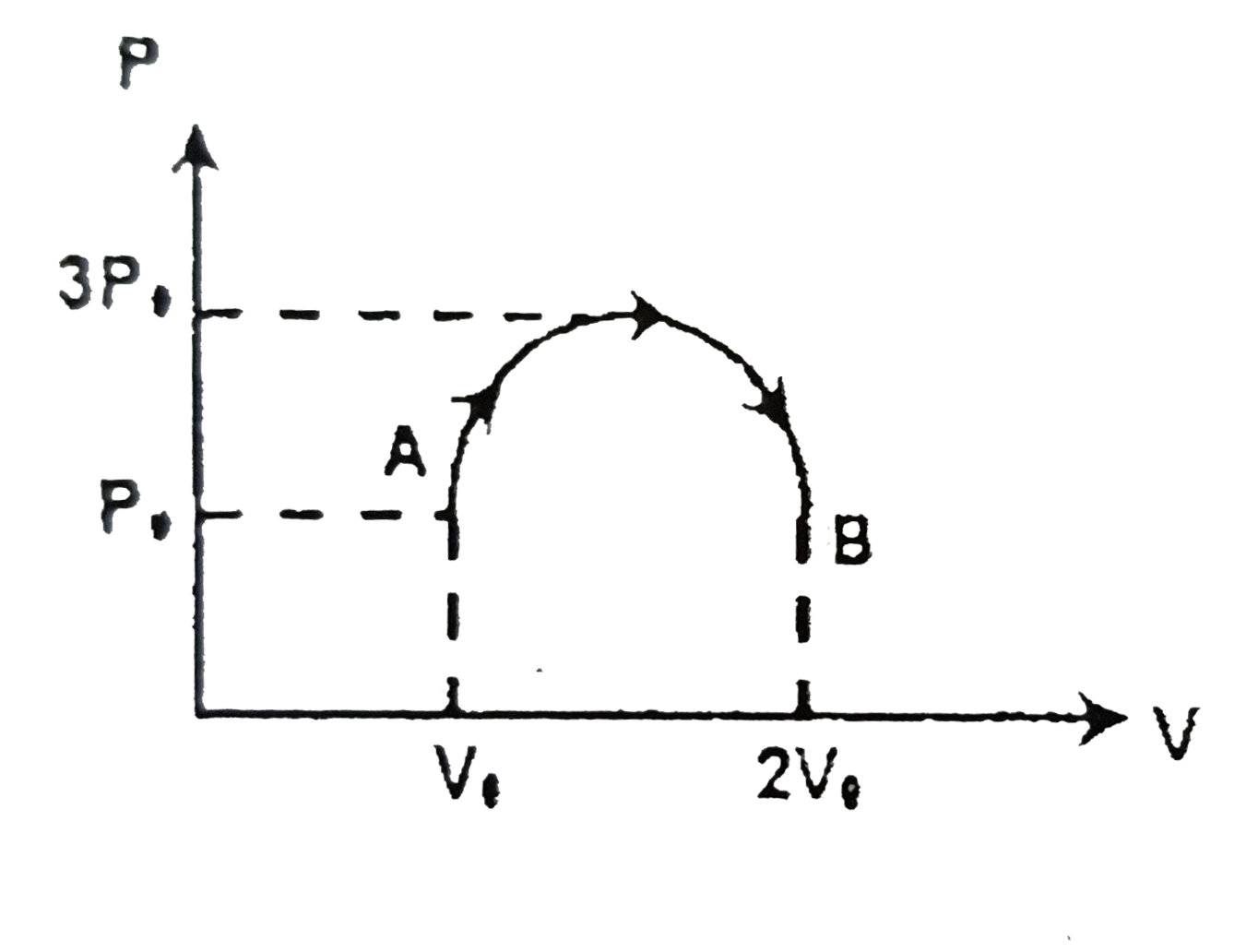 AP0V0(3+π2)BP0V0(2π−12)CP0V0(1+π)DP0V0(5+π2)
AP0V0(3+π2)BP0V0(2π−12)CP0V0(1+π)DP0V0(5+π2)Two moles of an ideal diatomic gas is taken through a process VT2= constant so that its temperature increases by ΔT=300K. The ratio (ΔUΔQ) of increase in internal energy and heat supplied to the gas during the process is
A2B3C4D5
In the polytropic process PV2 = constant, is the gas cooled'or heated with increase in volume.
View SolutionI mole of an ideal monoatomic gas is expanded till the temperature of the gas is doubled under the process V2T=contant. The initial temperature of the gas is 400K. In terms of R find total work done in the process.
View SolutionOne mole of an ideal gas with the adiabatic exponent γ goes through a polytropic process as a result of which the absolute temperature of the gas increases τ−fold. The polytropic constant equal n. Find the entropy increment of the gas in this process.
View SolutionOne mole of an ideal monoatomic has is taken through cyclic process ABC as shown in the graph with pressure and temperature as coordinate axes. Process AB is defined as PT = constant. Take universal gas constant to be R. Then:
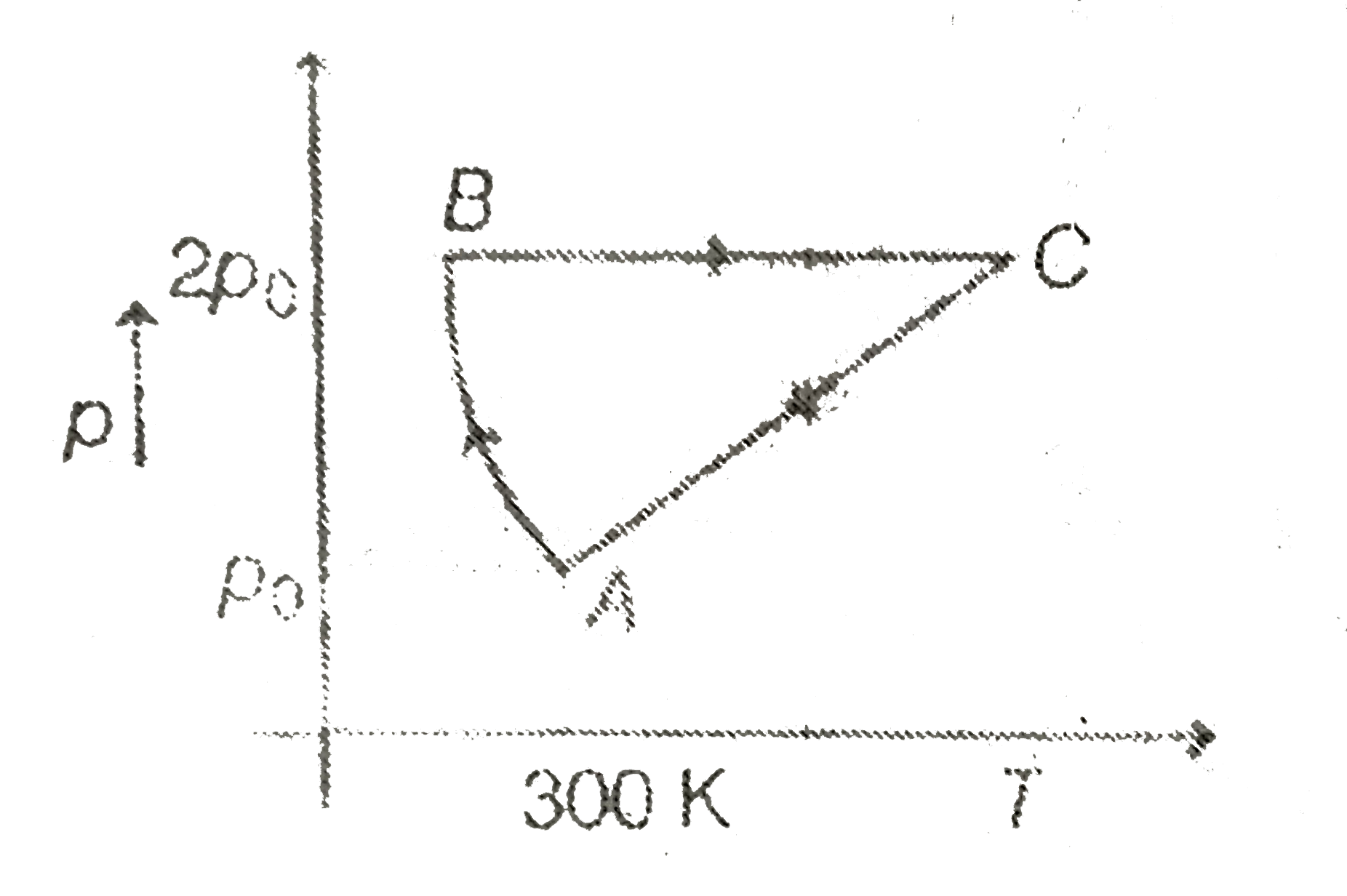
work done on gas in the process AB is
View SolutionOne mole of an ideal monoatomic has is taken through cyclic process ABC as shown in the graph with pressure and temperature as coordinate axes. Process AB is defined as PT = constant. Take universal gas constant to be R. Then:
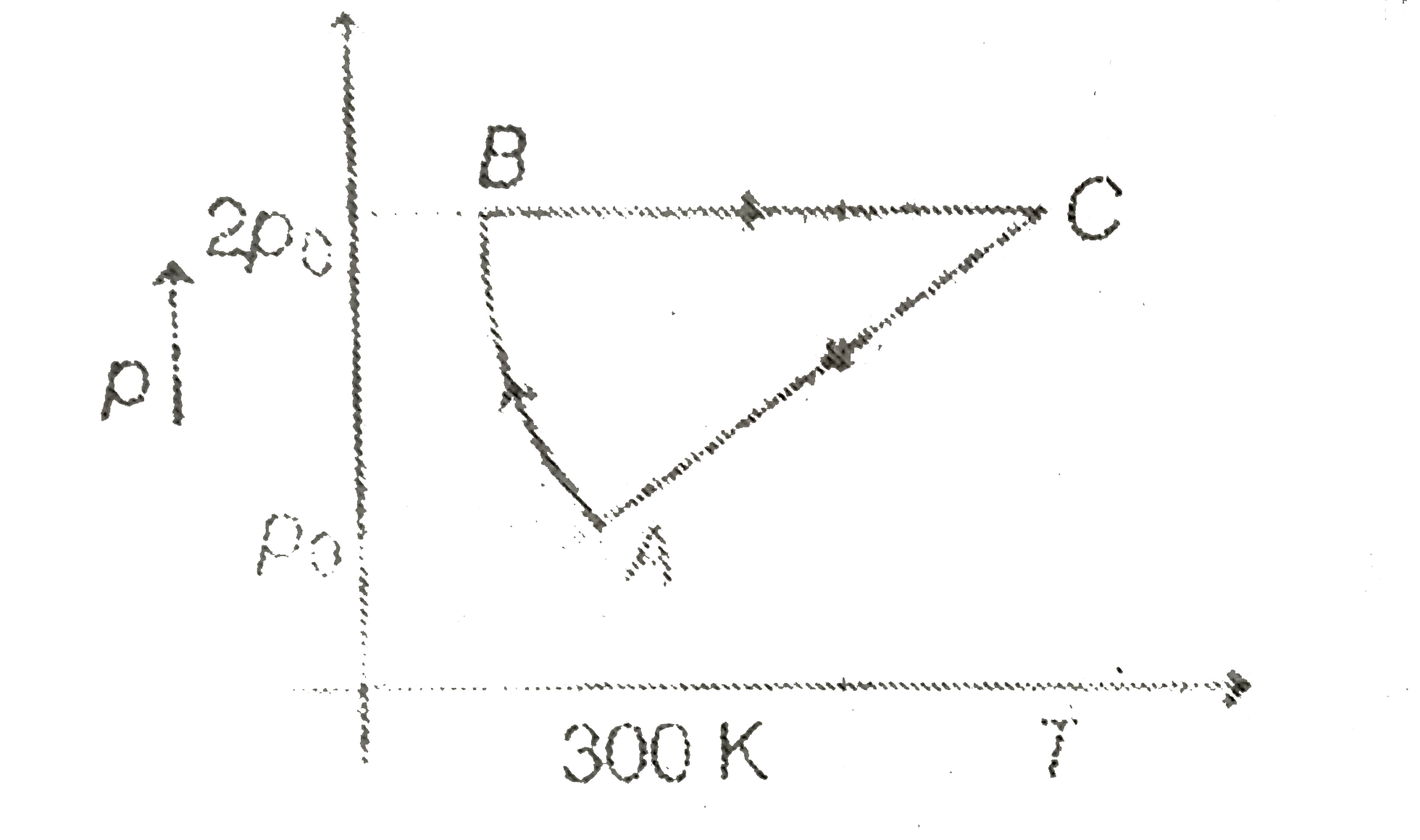
Change in internal energy of gas in the process CA is
View Solution

