Compressibility factor (Z) is plotted against pressure at different temperature for same gas
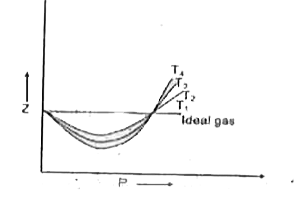
A
T4>T3>T2>T1
B
T1>T2>T3>T4
C
T1>T2>T3>T4
D
T1>T2>T3>T4
Text Solution
The correct Answer is:B
|
Similar Questions
Compressibility factor (Z=PVnRT) is plotted against pressure

Which is the correct order of liquefiability of the gases shown in the above graph ?
View SolutionCompressibility factor (Z=PVnRT) is plotted against pressure

Which is the correct order of liquefiability of the gases shown in the above graph ?
View Solution
Knowledge Check
The varation of compressibility factor Z fora real gas with pressure at different temperatures T1,T2andT3 is given as follows:
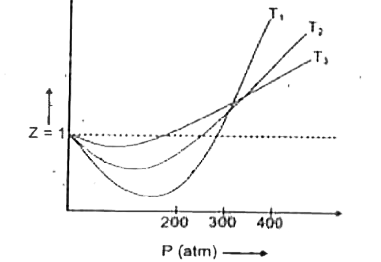
Which of the following statements are wrong?AT1=250K,T2=600KandT3=1100KBT1=1100K,T2=600KandT3=250KCAt low pressure and high temperature gas behaves ideally.DAt low pressure an ideal gas becomes real gas:For a real gas , the compressibility factor Z has different values at different temperatures and pressures . Which of the following is not correct under the given conditions ?
AZ < 1 at very low pressure .BZ > 1 at high pressure .CZ = 1 under all conditions .DZ = 1 at intermediate pressureFor a real gas , the compressibility factor Z has different values at different temperatures and pressures . Which of the following is not correct under the given conditions ?
AZ lt 1 at very low pressure .BZ gt 1 at high pressure .CZ = 1 under all conditions .DZ = 1 at intermediate pressure
The compressibility factor Z for the gas is given by
View SolutionThe compressibility factor Z for the gas is given by -
View SolutionFor a real gas the compressibility factor Z has different values at different temperatures and pressure. Which of the following is not correct under the given conditions ?
View SolutionThe compressibility factor Z for an ideal gas will be .........
View SolutionThe compressibility factor for a real gas at high pressure is .
View Solution

