Disintegration of radium takes place at an average rate of 2.24×1013 α-particles per minute. Each α-particle takes up 2 electrons from the air and becomes a neutral helium atom. After 420 days, the He gas collected was 0.5×10−3L measured at 300K and 750mm of mercury pressure. From the above data, calculate Avogadro's number.
No of α -particles (or) He formed =2.24×1013−1min
∴ No of He particles formed in 420days=2.24×1013×420×1440
=1.355×1019
Also at 27∘C and 750mm, He=0.5ml
Using PV=nRT
750760×0.51000=n×0.0821×300
⇒n=2.0×10−5 moles
2.0×10−5 moles of He=1.355×1019 particles of He
⇒1 mole of He =1.355×10192.0×10−5
=6.775×1023 particles
∴ Avagadro.s number =6.775×1023 particles/mol
Topper's Solved these Questions
- CHEMICAL KINETICS AND RADIOACTIVITYBook:FIITJEEChapter:CHEMICAL KINETICS AND RADIOACTIVITYExercise:Solved problem (Objective)
Explore 19 Videos
- CHEMICAL KINETICS AND RADIOACTIVITYBook:FIITJEEChapter:CHEMICAL KINETICS AND RADIOACTIVITYExercise:Comprehension I
Explore 3 Videos
- CHEMICAL KINETICS AND RADIOACTIVITYBook:FIITJEEChapter:CHEMICAL KINETICS AND RADIOACTIVITYExercise:Example
Explore 5 Videos
- CHEMICAL EQUILIBRIUMBook:FIITJEEChapter:CHEMICAL EQUILIBRIUMExercise:MATCHING TYPE QUESTIONS
Explore 3 Videos
- CHEMISTRY IN EVERY DAY LIFE Book:FIITJEEChapter:CHEMISTRY IN EVERY DAY LIFE Exercise:ASSIGNMENT PROBLEMS (OBJECTIVE) Level - II (Numerical Based Questions)
Explore 5 Videos
Similar Questions
The time of decay for thermonuclear reaction is given by t=5×t1/2. The relation between mean life T and time of decay t is given by
View SolutionUnstable nuclei attain stability through disintergration. The nuclear stability is related to neutron- proton ratio (n/p).n/p ratio for stable nuclei lies quite close to unity for elements with low atomic numbers (20 or less) but it is more than one for nuclei having higher atomic numbers. Nuclei having n/p ratio either very high or low undergo nuclear transformation.
(i) When n/p ratio is higher than required for stability, the nuclei have the tendency to emit β-rays, i.e. a neutron is converted into a proton.
(ii) When n/p ratio is lower than required for stability, the nuclei increase the ratio, either by emitting α-particle or by emitting a positron or by K-electron capture.
2713Al is a stable isotope , 2913Al is expected to disintegrate by-
View SolutionThe progress of the reaction given below,
CH3COOC2H5+H2OH+−−→CH3COOH+C2H5OH
can be followed by measuring the concentration of acid (HCl acid used as catalyst plus acetic acid formed during the reaction) by means of alkali titration. Find the volume of alkali (NaOH) needed for the end point that will increase with time.
View Solution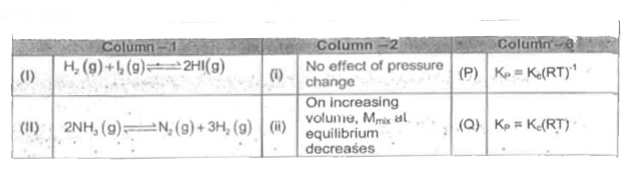

Which of the following combination is correct ?
View SolutionHow many of the following are water soluble vitamins ?
A, B, C, D , K, E
View SolutionCalculate the number of neutrons in the remaining atoms after the emission of an alpha particle from .92U238 atom.
View SolutionThe number of alpha particles scattered at 60∘ is 100 per minute in an alpha particle scattering experiment. Calculate the number of alpha particles scattered per minute at 90∘.
View SolutionAfter emission of one α particle followed by one β-particle from 23892X, the number of neutrons in the atom will be
View SolutionWhich does not take place by α disintegration ?
View SolutionHow many alpha particles are emitted per second by 1 microgram of radium
View SolutionIf 1 microgram of radium has disintegrated for 500 years, how many alpha particles will be emitted per second
View SolutionWhat is the difference between α - particle and helium atom ?
View SolutionIf energy required to remove one of the two electrons from He atom is 29.5 eV , then what is the value of energy required to convert a helium atom into alpha -particle?
View SolutionIn an α-particle scattering experiment , 100 particles are scattered per minute at an angle of 60∘ . Calculate the number of particles per minute scattered at an angle of 30∘
View SolutionIn an alpha -particle scattering experiment, the number of particles scattered at an angle of 90^@ is 100 per minute. Calculate the number of particles scattered at an angle of 60^@
View Solution

