A catalyst increases the rate of chemical reaction by providing an alternative reaction pathway which lowers the activation energy than the reaction pathway in its absence. A catalyst is not consumed and therefore does not appear in the chemical equation for the reaction. A homogenous catalyst is in the same phase as the ractants whilst a heterogeneous catalyst is in a different phase.
The complex [Co(NH3)3F]2++H2O→[Co(NH3)5(H2O+F−]3+
and Rate =k[complex]a.[H+]b
The reaction is acid catalysed , i.e. [H+] does not change during the reaction . Thus, rate =k'[complex] where, k'=[H+]b
![A catalyst increases the rate of chemical reaction by providing an alternative reaction pathway which lowers the activation energy than the reaction pathway in its absence. A catalyst is not consumed and therefore does not appear in the chemical equation for the reaction. A homogenous catalyst is in the same phase as the ractants whilst a heterogeneous catalyst is in a different phase. The complex [Co(NH(3))(3)F]^(2+)+H(2)Oto[Co(NH(3))(5)(H(2)O+F^(-)]^(3+) and Rate =k[](https://d10lpgp6xz60nq.cloudfront.net/physics_images/FIITJEE_CHE_MB_02_C02_E05_077_Q01.png)
For the reaction , CH3COCH3+I2H+−−→ Products
The rate is governed by the expression dxdt=k[Acetone][H+]
The order w.r.t. iodine is
0
1
2
3
The correct Answer is:A
Topper's Solved these Questions
- CHEMICAL KINETICS AND RADIOACTIVITYBOOK - FIITJEECHAPTER - CHEMICAL KINETICS AND RADIOACTIVITYEXERCISE - Assignment problems (Objective) Level-II ( Comprehension-II)
3 Videos
- CHEMICAL KINETICS AND RADIOACTIVITYBOOK - FIITJEECHAPTER - CHEMICAL KINETICS AND RADIOACTIVITYEXERCISE - Assignment problems (Objective) Level-II (Comprehension-III )
4 Videos
- CHEMICAL KINETICS AND RADIOACTIVITYBOOK - FIITJEECHAPTER - CHEMICAL KINETICS AND RADIOACTIVITYEXERCISE - Assignment problems (Objective) Level-II
21 Videos
- CHEMICAL EQUILIBRIUMBOOK - FIITJEECHAPTER - CHEMICAL EQUILIBRIUMEXERCISE - MATCHING TYPE QUESTIONS
3 Videos
- CHEMISTRY IN EVERY DAY LIFE BOOK - FIITJEECHAPTER - CHEMISTRY IN EVERY DAY LIFE EXERCISE - ASSIGNMENT PROBLEMS (OBJECTIVE) Level - II (Numerical Based Questions)
5 Videos
Similar Questions
Knowledge Check
A catalyst increases the rate of chemical reaction by providing an alternative reaction pathway which lowers the activation energy than the reaction pathway in its absence. A catalyst is not consumed and therefore does not appear in the chemical equation for the reaction. A homogenous catalyst is in the same phase as the ractants whilst a heterogeneous catalyst is in a different phase.
The complex [Co(NH3)3F]2++H2O→[Co(NH3)5(H2O+F−]3+
and Rate =k[complex]a.[H+]b
The reaction is acid catalysed , i.e. [H+] does not change during the reaction . Thus, rate =k'[complex] where, k'=[H+]b

Overall order of reaction isA1B2C0D12A catalyst increases the rate of chemical reaction by providing an alternative reaction pathway which lowers the activation energy than the reaction pathway in its absence. A catalyst is not consumed and therefore does not appear in the chemical equation for the reaction. A homogenous catalyst is in the same phase as the ractants whilst a heterogeneous catalyst is in a different phase.
The complex [Co(NH3)3F]2++H2O→[Co(NH3)5(H2O+F−]3+
and Rate =k[complex]a.[H+]b
The reaction is acid catalysed , i.e. [H+] does not change during the reaction . Thus, rate =k'[complex] where, k'=[H+]b
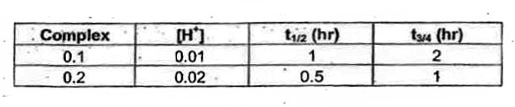
From the above data, value of a is :A1B2C0D12A catalyst increases the rate of a chemical reaction by
AIncreasing the activation energyBDecreasing the activation energyCReacting with reactantsDReacting with products
A catalyst increases the rate of a chemical reaction by
View SolutionA catalyst increases the rate of a chemical reaction by
View SolutionA catalyst increases the rate of a chemical reaction by -
View SolutionA catalyst increases the rate of a chemical reaction by
View SolutionA catalyst increases the rate of chemical reaction by providing an alternative reaction pathway which lowers the activation energy than the reaction pathway in its absence. A catalyst is not consumed and therefore does not appear in the chemical equation for the reaction. A homogenous catalyst is in the same phase as the ractants whilst a heterogeneous catalyst is in a different phase.
The complex [Co(NH3)3F]2++H2O→[Co(NH3)5(H2O+F−]3+
and Rate =k[complex]a.[H+]b
The reaction is acid catalysed , i.e. [H+] does not change during the reaction . Thus, rate =k'[complex] where, k'=[H+]b

The value of activation energy for a chemical reaction primarily depends on
View Solution

