STATEMENT-1: The highest oxidation state of chromium in its compounds is +6. and STATEMENT-2: Chromium atom has only six electrons in ns and (n-1)d orbitals.
STATEMENT - 1 is True, STATEMENT-2 is True, STATEMENT -2 is correct explanation for STATEMENT-1
STATEMENT - 1 is True, STATEMENT-2 is True, STATEMENT -2 is NOT a correct explanation for STATEMENT-1
STATEMENT - 1 is True, STATEMENT-2 is False
STATEMENT - 1 is False, STATEMENT-2 is True
The correct Answer is:A
Topper's Solved these Questions
- TRANSITION ELEMENTS & COORDINATION COMPOUNDSBOOK - FIITJEECHAPTER - TRANSITION ELEMENTS & COORDINATION COMPOUNDSEXERCISE - MATRIX-MATCH THE FOLLOWING QUESTIONS
10 Videos
- TRANSITION ELEMENTS & COORDINATION COMPOUNDSBOOK - FIITJEECHAPTER - TRANSITION ELEMENTS & COORDINATION COMPOUNDSEXERCISE - SINGLE INTEGER ANSWER TYPE QUESTIONS
8 Videos
- TRANSITION ELEMENTS & COORDINATION COMPOUNDSBOOK - FIITJEECHAPTER - TRANSITION ELEMENTS & COORDINATION COMPOUNDSEXERCISE - ASSIGNMENT PROBLEMS (COMPREHENSION -IV)(Level- II)(Read the above paragraph carefully and answer the questions given below it)
8 Videos
- THERMODYNAMICS AND THERMOCHEMISTRY BOOK - FIITJEECHAPTER - THERMODYNAMICS AND THERMOCHEMISTRY EXERCISE - SINGLE INTEGER ANSWER TYPE QUESTIONS
5 Videos
Similar Questions
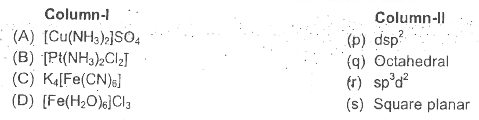
View SolutionWhat will be the total number of possible isomers of the compound:[CuII(NH3)4](PtIICl4]
View SolutionVolumetric titrations involving KMnO4, are carried out only in presence of dilute H2SO4 but not in the presence of HCI or HNO3 This is because oxygen produced from KMnO4 + dil: H2SO4, is used only for oxidizing the reducing agent. Moreover, H2SO4 does not give any oxygen of its own to oxidize the reducing agent. In case HCI is used, the oxygen produced from KMnO4+HCI is partly used up to oxidize HCI and in case HNO3 is used, it itself acts as oxidizing agent and partly oxidizes the reducing agent. KMnO4, in various mediums gives following productsMn7+H+−−→Mn2+
Mn7+H2O−−→Mn4+
Mn7+−−→OH−Mn6+
QThe equivalent weight of KMnO4, in the given reaction isKMnO4→K2MnO4+MnO2+O2
View Solution4.48L of an ideal gas at STP requires 12 cal to raise its temperature by 15∘C at constant volume. The CP of the gas is
View SolutionThe atomic number of chromium is 24 Its electronic coefigueration in ground state in 1s22s22p63s24s13d5 .Chromium atom by using 3 electron from Cr2+ .A chromium atom contain 17% morte neutron than the proton. Now answer the following questions
The number of electron having n=3 and m1=0 in chromium atom is
View SolutionStatement -1:Aluminothermy is used for extraction of chromium from chromium oxide. Statement-2: Aluminium is reducing agent.
View SolutionChromium has the lowest oxidation state in
View SolutionChromium has the lowest oxidation state in
View SolutionSTATEMENT-1 : Chromium atom has electronic configuration [Ar]3d54s1 .
and
STATEMENT-2: Atomic number of chromium is 24 .
View SolutionWhat is the highest oxidation state of chromium ?
View SolutionThe element having the lowest atomic number and a ground state electronic configuration of (n−1)d6ns2 is placed in:
View Solutionनिम्न में से किस यौगिक में क्रोमियम की ऑक्सीकरण अवस्था +6 है
View Solutionअभिकथनः क्रोमियम की अधिकतम ऑक्सीकरण अवस्था इसके यौगिकों में + 6 होती है।
तर्कः क्रोमियम के ns एवं (n-1)d कक्षकों में केवल छह इलेक्ट्रॉन होते हैं।
View SolutionChromium atom has six unpaired electrons. Substantiate.
View Solution(A) The highest oxidation state of chromium in its compounds is + 6
(R) Chromium atom has only six electrons in ns and (n-1)d orbitals.
View SolutionThe atomic number of chromium is 24 Its electronic configuration in ground state in 1s22s22p63s23p64s13d5 Now answer the following questions
The number of electron having n=3 and m1=0 in chromium atom is
View Solution

