Arrange the following groups in decreasing activating order :
(i) −NR2
(ii) −Me
(iii) NHAc
(iv) −OH
A
I>IV>III>II
B
II>IV>III>I
C
III>II>IV>I
D
IV>I>III>II
Text Solution
The correct Answer is:A
|
Similar Questions
Knowledge Check
Arrange the following groups in order of decreasing +M effect
(i) −⊕O (ii) −NH2 (iii) −OH (iv) −NHCOCH3Ai>ii>iii>ivBiv>iii>ii>iCi>iii>ii>ivDi>iv>iii>iiArrange the following oxides in decreasing acidic order
SiO2 (I) CO2(II) SO3(III) N2O5(IV)AIII>IV>II>IBIV>III>II>ICIV>II>III>IDIII>II>IV>IArrange the following in their decreasing order of acidity.
(I)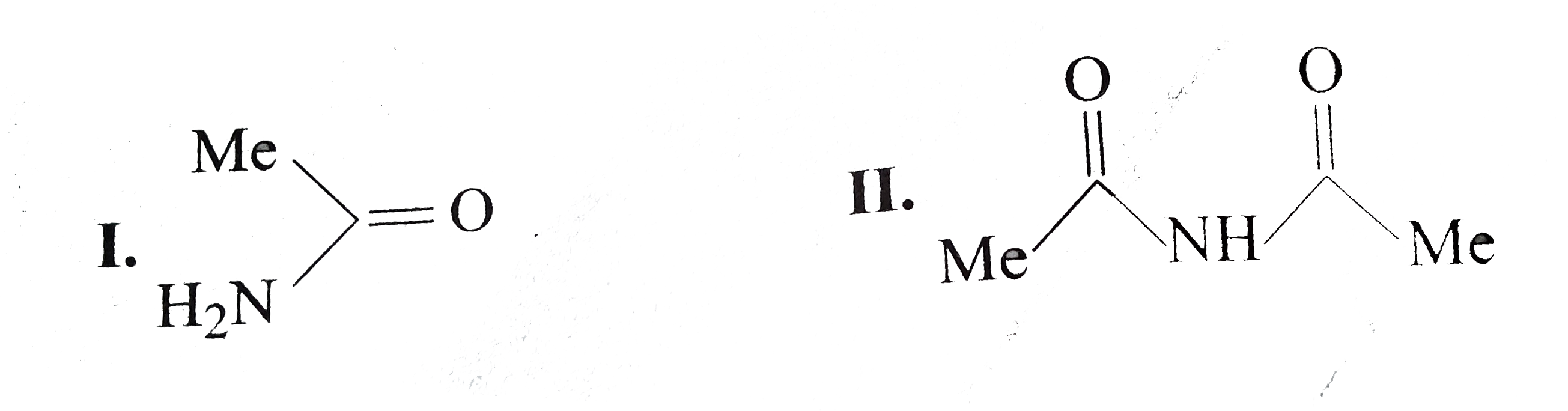
(III)
(IV) NH3.AIII>IV>I>IIBI>II>III>IVCIV>III>II>IDII>III>I>IV
Arrange the following oxides in decreasing acidic order
SiO2 (I) CO2(II) SO3(III) N2O5(IV)
View SolutionArrange the following groups in the increasing order of +M :
(i) −ICl,−F,−Br (ii) −NH2,−OH,−OΘ
View SolutionArrange the following groups in their decreasing order of -I effect.
I. NO2 II. SO3H III. F IV. NH2
The correct choice is :
View SolutionArrange the following groups in their decreasing order of -I effect.
I. NO2 II. SO3H III. F IV. NH2
The correct choice is :
View SolutionArrange the following compounds in the order of decreasing acidity.
I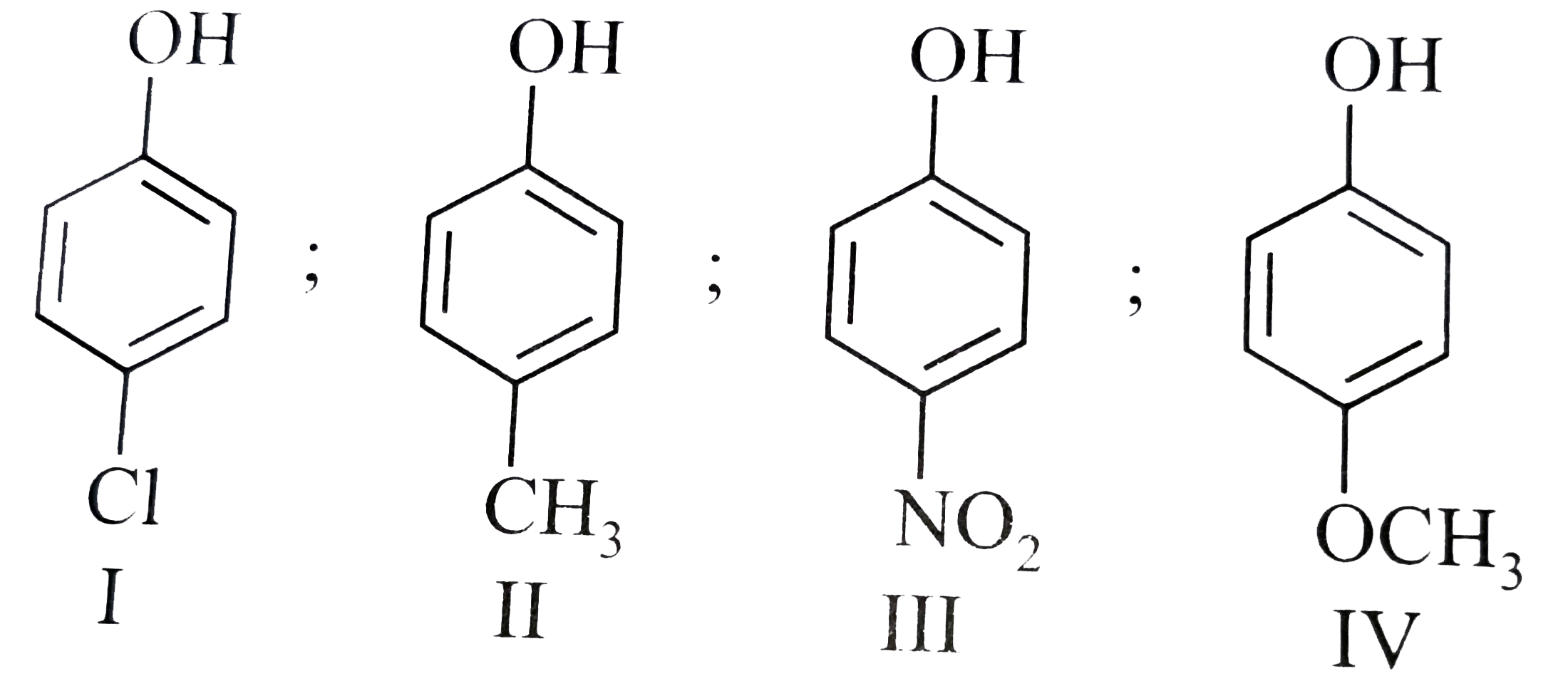 II
II  III
III  IV
IV 
View Solution


 (ii)
(ii) 