A crystal is made of particle X,Y and Z 'X' forms +FCC packing+. 'Y' occupies all the octahedral voids of X and Z occupies all tetrahedral voiods of X. If all the particles along one body diagonal are removed, then the formula of the crystal would be?
XYZ2
X2YZ2
X8Y4Z5
none of these
The correct Answer is:C
Topper's Solved these Questions
- CHEMICAL ENERGETICS BOOK - FIITJEECHAPTER - CHEMICAL ENERGETICS EXERCISE - ASSIGNMENT PROBLEMS (SECTION (II) JEE ADVANCED WITH SINGLE CORRECT CHOICE)
84 Videos
- CHEMICAL ENERGETICS BOOK - FIITJEECHAPTER - CHEMICAL ENERGETICS EXERCISE - MORE THAN ONE CORRECT OPTION
32 Videos
- CHEMICAL ENERGETICS BOOK - FIITJEECHAPTER - CHEMICAL ENERGETICS EXERCISE - NUMERICAL BASED QUESTIONS
2 Videos
- CARBOXYLIC ACIDS AND ITS DERIVATIVESBOOK - FIITJEECHAPTER - CARBOXYLIC ACIDS AND ITS DERIVATIVESEXERCISE - SINGLE INTEGER ANSWER TYPE QUESTIONS
1 Video
- CHEMICAL EQUILIBRIUMBOOK - FIITJEECHAPTER - CHEMICAL EQUILIBRIUMEXERCISE - MATCHING TYPE QUESTIONS
3 Videos
Similar Questions
A crystal is made of particles X,Y and Z.X form fcc packing . Y occupies all the octahedral void of X and Z occupies all the tetrahedral voids of X . If all the particles along one body diagonal are removed then the formula of the crystal would be:
View SolutionA crystal is made of particles X,Y and Z.X form fcc packing . Y occupies all the octahedral void of X and Z occupies all the tetrahedral voids of X . If all the particles along one body diagonal are removed then the formula of the crystal would be:
View Solution
Knowledge Check
A crystal is made up of particals X, Y, and Z. X froms f packing. Y occupies all octahedral voids of X and Z occupies all tetrahedral voids of X. If all the particles along one body diagonal are removed. Then the fromula of the crystal would be
AXYZ2BX2YZ2CX8Y4Z5DX5Y4Z8A crystal is made up of particles A. B and C. A forms fcc packing, B occupies all octahedral voids and C occupies all tetrahedral voids. If all the particles along one body diagonal are removed, then the formula of the crystal would be
AABC2BA2BC2CA8B4C5DA5B4C8A crystal is made of particles X and Y.X form fcc packing and Y occupies all the octahedral voids . If all the particles along one body diagonal are removed then the formula of the crystal would de :
AX4Y3BX5Y5CX4Y5DNone of these
A crystal is made of particles X,Y and Z.X form fcc packing . Y occupies all the octahedral void of X and Z occupies all the tetrahedral voids of X . If all the particles along one body diagonal are removed then the formula of the crystal would be:
View SolutionA crystal is made of particles X and Y.X form fcc packing and Y occupies all the octahedral voids . If all the particles along one body diagonal are removed then the formula of the crystal would de :
View SolutionA crystal is made of particles A and B . From fcc packing and B occupies all the octahedral voids . If all the particle along the plane as shown in figure are removed , then, the formula of the crystal would be :
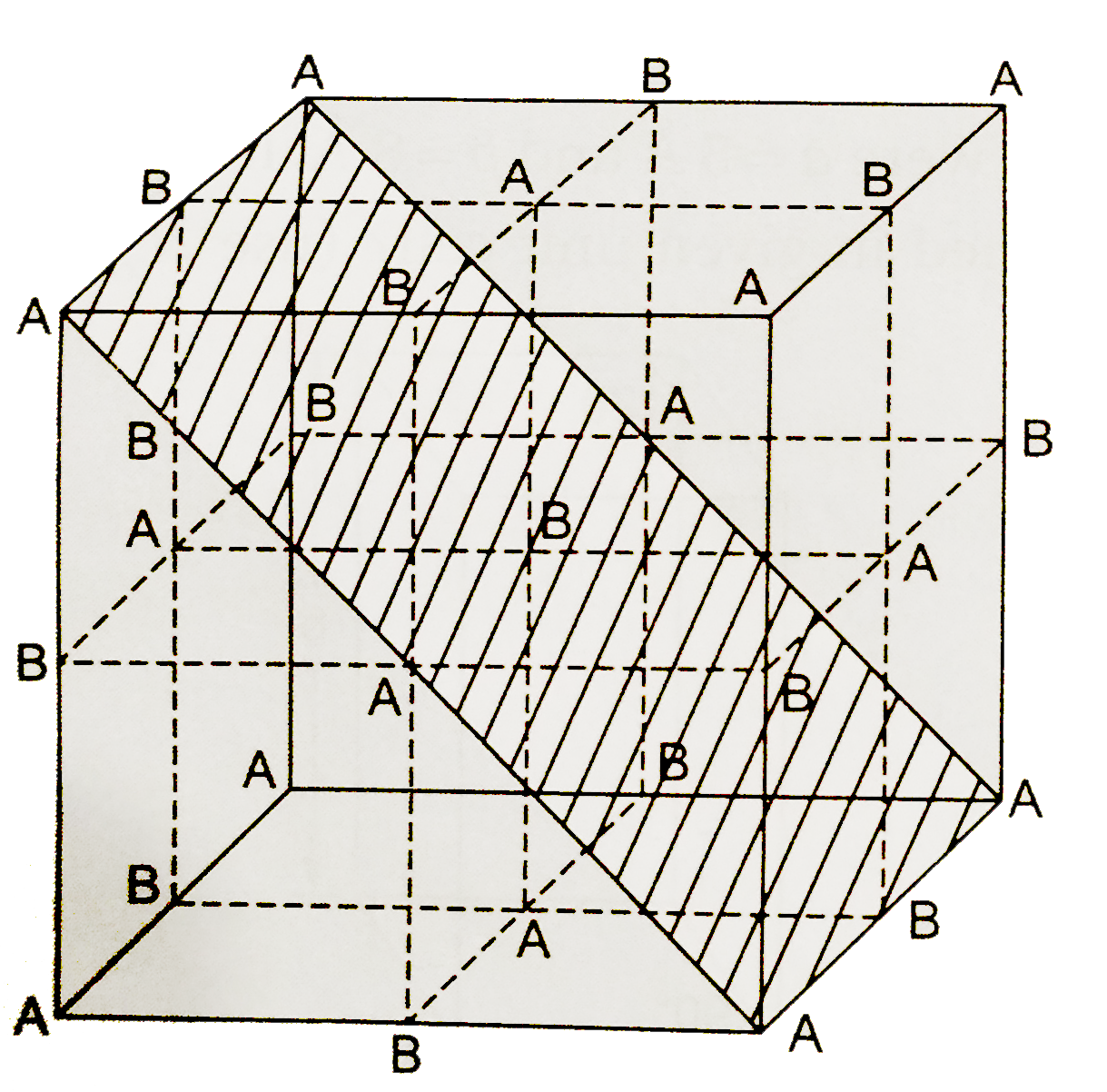
View SolutionA crystal is made of particles X and Y. X forms FCC packing and Y occupies all the octahedral voids. Find the formula of the crystal if all the particles along one body diagonal are removed.
View SolutionA forms ccp lattice B occupy half of the octahedral voids and O occupy all the tetrahedral voids. Calculate formula-
View Solution

