Number of 3 central 2 electron bonds present in B2H6 is
The correct Answer is:2
Topper's Solved these Questions
- CHEMICAL ENERGETICS BOOK - FIITJEECHAPTER - CHEMICAL ENERGETICS EXERCISE - NUMERICAL BASED QUESTIONS
2 Videos
- CHEMICAL ENERGETICS BOOK - FIITJEECHAPTER - CHEMICAL ENERGETICS EXERCISE - NUMERICAL BASED PROBLEMS
12 Videos
- CARBOXYLIC ACIDS AND ITS DERIVATIVESBOOK - FIITJEECHAPTER - CARBOXYLIC ACIDS AND ITS DERIVATIVESEXERCISE - SINGLE INTEGER ANSWER TYPE QUESTIONS
1 Video
- CHEMICAL EQUILIBRIUMBOOK - FIITJEECHAPTER - CHEMICAL EQUILIBRIUMEXERCISE - MATCHING TYPE QUESTIONS
3 Videos
Similar Questions
Knowledge Check
Number of unpaired electrons present in[Ni(H2O)6]3+
ATwoBOneCFourDThreeNumber of unpaired electrons present in [Ni(H2O)6]2+
ATwoBOneCFourDThreeNumber of co-ordinate bond present in H2SO4 molecule is
A0B1C2D3
Number of coordinate covalent bonds present in [AlCl(H2O)5]2+ is N; value of 50×N is _______
View SolutionThe number of 2-centre-2-electron and 3-centre-2-electron bonds in B2H6, respectively, are
View SolutionThe number of 2-centre-2-electron and 3-centre-2-electron bonds in B2H6, respectively, are
View SolutionThe number of 2-centre-2-electron and 3-centre-2-electron bonds in B2H6, respectively, are
View SolutionWhich of the following statements are correct regarding diborane?
I. Two bridged hydrogen atoms and two boron atoms in one plane.
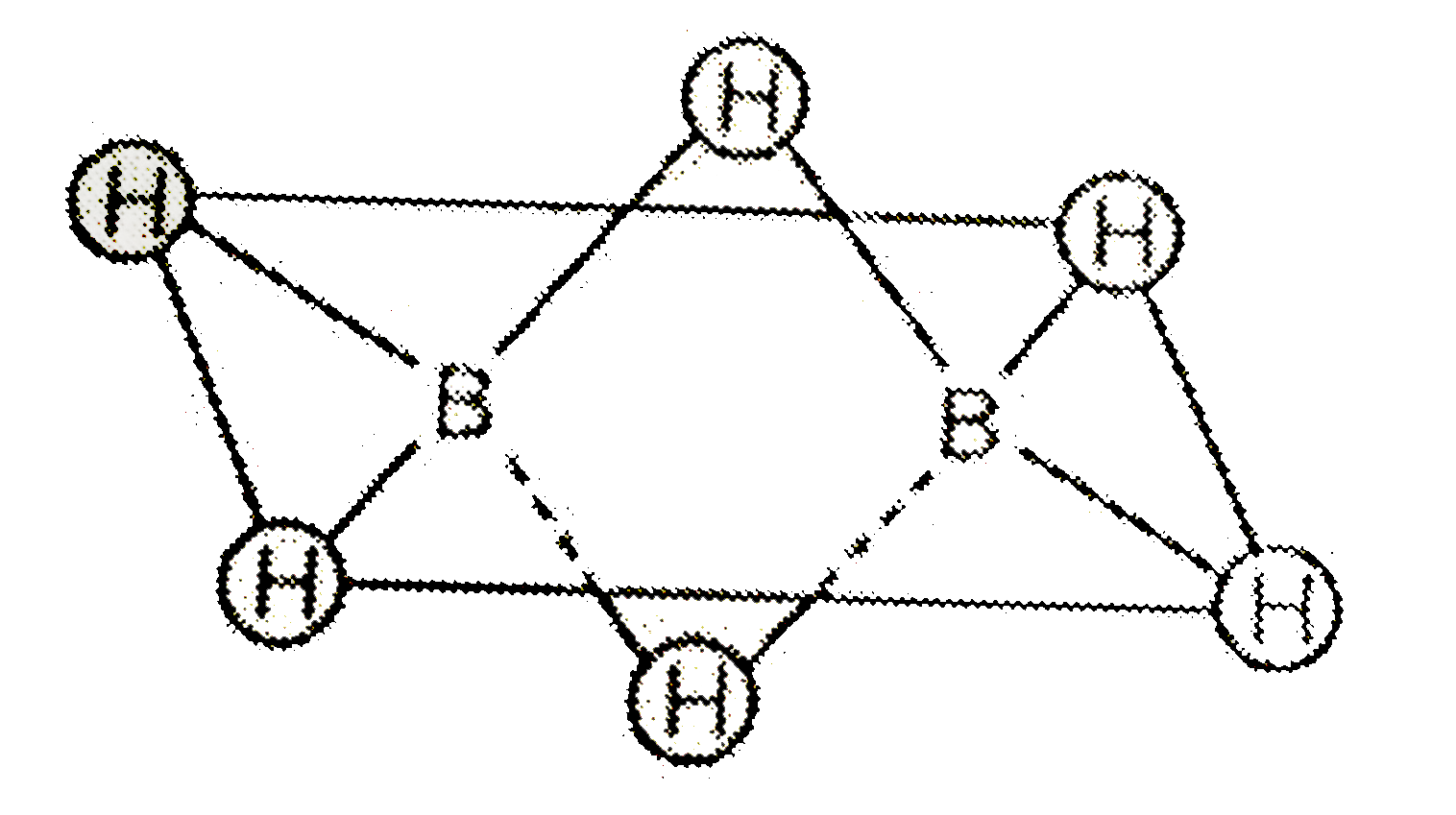
II. Out of six B-H bond, two bonnds can be described in terms of 3 centre-2 electron bonds.
III. Out of six B-H bonds, four B-H bonds can be described in terms of 3 centre-2 electron bonds.
IV. Four terminal B-H bonds are two centre electrons regular bonds.
View Solution

