Which of the following reaction will not give methane?
CH3COONaNaOH+CaO−−−−−−−→Δ
BeC2+H2OΔ−−→
Al4C3+H2OΔ−−→
All of those
The correct Answer is:2
To determine which of the following reactions will not produce methane, we will analyze each option step by step.
1. Identify the Reactions: We have three reactions to analyze:
- Sodium acetate with soda lime.
- Beryllium carbide with water.
- Aluminum carbide with water.
2. Reaction of Sodium Acetate with Soda Lime:
- Sodium acetate (CH₃COONa) is heated with soda lime (a mixture of NaOH and CaO).
- This process involves decarboxylation, where the carboxylic acid group is removed, releasing carbon dioxide (CO₂).
- The reaction can be represented as:
CH3COONa+NaOHΔ−→CH4+CO2
- Conclusion: This reaction produces methane (CH₄).
3. Reaction of Beryllium Carbide with Water:
- Beryllium carbide (Be₂C) reacts with water (H₂O).
- The reaction produces acetylene (C₂H₂) and beryllium hydroxide (Be(OH)₂).
- The reaction can be represented as:
Be2C+2H2O→C2H2+Be(OH)2
- Conclusion: This reaction does not produce methane; it produces acetylene instead.
4. Reaction of Aluminum Carbide with Water:
- Aluminum carbide (Al₄C₃) reacts with water.
- The reaction produces methane and aluminum hydroxide (Al(OH)₃).
- The reaction can be represented as:
Al4C3+12H2O→3CH4+4Al(OH)3
- Conclusion: This reaction produces methane (CH₄).
5. Final Decision:
- From the analysis:
- Sodium acetate with soda lime produces methane.
- Beryllium carbide with water does not produce methane.
- Aluminum carbide with water produces methane.
- Therefore, the reaction that will not give methane is the reaction of Beryllium carbide with water.
Answer:
The reaction that will not give methane is Beryllium carbide with water.
---
- Sodium acetate with soda lime.
- Beryllium carbide with water.
- Aluminum carbide with water.
- Sodium acetate (CH₃COONa) is heated with soda lime (a mixture of NaOH and CaO).
- This process involves decarboxylation, where the carboxylic acid group is removed, releasing carbon dioxide (CO₂).
- The reaction can be represented as:
CH3COONa+NaOHΔ−→CH4+CO2
- Conclusion: This reaction produces methane (CH₄).
- Beryllium carbide (Be₂C) reacts with water (H₂O).
- The reaction produces acetylene (C₂H₂) and beryllium hydroxide (Be(OH)₂).
- The reaction can be represented as:
Be2C+2H2O→C2H2+Be(OH)2
- Conclusion: This reaction does not produce methane; it produces acetylene instead.
- Aluminum carbide (Al₄C₃) reacts with water.
- The reaction produces methane and aluminum hydroxide (Al(OH)₃).
- The reaction can be represented as:
Al4C3+12H2O→3CH4+4Al(OH)3
- Conclusion: This reaction produces methane (CH₄).
- From the analysis:
- Sodium acetate with soda lime produces methane.
- Beryllium carbide with water does not produce methane.
- Aluminum carbide with water produces methane.
- Therefore, the reaction that will not give methane is the reaction of Beryllium carbide with water.
The reaction that will not give methane is Beryllium carbide with water.
Similar Questions
Knowledge Check
Which of the following reaction will not give name
AR−X+NH3BR−CH=N−OH+Na+ alcoholCR−CN+H2ODR−CN+H2Which of the following reaction will not give ether as a major product ?
ACH3CH2Cl+Ag2O(dry)B(CH3)3CCl+CH3CH2O−Na+→C DCH3Cl++Na−O−CH3| C | CH3−CH3→
DCH3Cl++Na−O−CH3| C | CH3−CH3→Which of the following reactions will not give the anhydrous AlCl3 ?
ABy heating AlCl3⋅6H2OBBy passing dry HCl gas on heated aluminium powderCBy passing dry chloine gas on heated aluminium powderDBy passing dry chlorine gas over a heated mixture of alumina and coke
Which of the following reactions will not give propane ?
View SolutionWhich of the following reactions will not give propane ?
View SolutionWhich of the following reactions will not give p- aminoazobenzene?
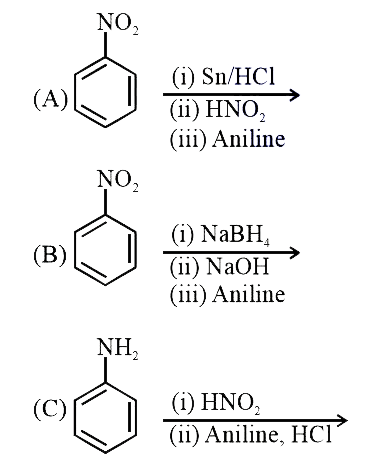
View SolutionWhich of the following reaction will give an alkyne?
View SolutionWhich of the following reaction will not give picric acid?
View Solution
