Natural rubber is a polymer of isoprene(2-methylbuta-1, 3-diene). If natural rubbber is treated with O3 followed by Zn/H2O, the final product will be
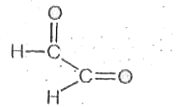
CH3−O∣∣C−CH2−CH2−CHO
CH3−CH2−O∣∣C−CH2−CHO
The correct Answer is:2
To solve the problem, we need to follow these steps:
Step 1: Understand the structure of isoprene
Isoprene, or 2-methylbuta-1,3-diene, has the following structure:
CH2=C(CH3)−C=CH2
This structure consists of a five-carbon chain with two double bonds and a methyl group on the second carbon.
Step 2: Polymerization of isoprene
Natural rubber is a polymer made up of many isoprene units. The polymerization of isoprene involves the addition of multiple isoprene monomers to form long chains.
Step 3: Ozonolysis of natural rubber
When natural rubber (a polymer of isoprene) is treated with ozone (O3), it undergoes ozonolysis. This reaction cleaves the double bonds in the polymer, resulting in the formation of carbonyl compounds.
Step 4: Reduction of ozonolysis products
After ozonolysis, the product is treated with zinc (Zn) and water (H2O), which reduces the ozonolysis products. The ozonolysis of isoprene results in the formation of two types of carbonyl compounds: aldehydes and ketones.
Step 5: Identify the final products
The ozonolysis of isoprene will yield:
- Acetone (CH3COCH3)
- Formaldehyde (HCHO)
However, since we are dealing with the polymer of isoprene, the final products after ozonolysis and reduction will be:
- Acetaldehyde (CH3CHO)
- Formaldehyde (HCHO)
Thus, the final products will be:
- One molecule of acetaldehyde (CH3CHO)
- One molecule of formaldehyde (HCHO)
Final Answer
The final products after treating natural rubber with O3 followed by Zn/H2O will be:
- Acetaldehyde (CH3CHO) and Formaldehyde (HCHO)
---
Isoprene, or 2-methylbuta-1,3-diene, has the following structure:
Natural rubber is a polymer made up of many isoprene units. The polymerization of isoprene involves the addition of multiple isoprene monomers to form long chains.
When natural rubber (a polymer of isoprene) is treated with ozone (O3), it undergoes ozonolysis. This reaction cleaves the double bonds in the polymer, resulting in the formation of carbonyl compounds.
After ozonolysis, the product is treated with zinc (Zn) and water (H2O), which reduces the ozonolysis products. The ozonolysis of isoprene results in the formation of two types of carbonyl compounds: aldehydes and ketones.
The ozonolysis of isoprene will yield:
- Acetone (CH3COCH3)
- Formaldehyde (HCHO)
- Acetaldehyde (CH3CHO)
- Formaldehyde (HCHO)
- One molecule of acetaldehyde (CH3CHO)
- One molecule of formaldehyde (HCHO)
The final products after treating natural rubber with O3 followed by Zn/H2O will be:
- Acetaldehyde (CH3CHO) and Formaldehyde (HCHO)
Similar Questions
Knowledge Check
Natural rubber is all cis polymer o fisoprene, what is the name of all trans polymer of same monomer ?
ANeopreneBGutta-perchaCBuna-NDBuna-SHexa-2,4-diene on ozonolysis by using O3/(C)Cl4 followed by Zn/H2O gives:
AacetaldehydeBglyoxalCformaldehydeDpropanaldehydeIsoprene(2-methyl 1,3-butadiene) is a basic unit of the polymer rubber
Abuna N rubberBbuna S rubberCneoprene rubberDnatural rubber
An alkene CH3CH=CH2 is treated with B2H6 in presence of H2O2 . The final product formed is
View SolutionWhen SO3 is treated with D2O, the products are :
View Solution
What product will be obtained if 'W' is treated with C6H5MgBr followed by D3O⊕ and then by heating ?
View SolutionAssertion (A): The IUPAC name of isoprene is 2-methyl buta-1,3-diene.
Reason (R): Isoprene unit is a monomer of natural rubber
View SolutionAcetylene on ozonolysis with
(O3+Zn/H2O) gives
View Solution

