In which of the following dehydration by conc. H2SO4 no rearrangement is favourable ?
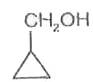
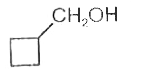
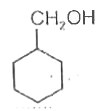
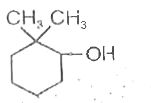
The correct Answer is:1
To determine which dehydration by concentrated H2SO4 does not favor rearrangement, we need to analyze the carbocation stability formed during the dehydration process. The dehydration of alcohols typically involves the following steps:
Step 1: Protonation of Alcohol
The first step in the dehydration mechanism is the protonation of the alcohol by concentrated sulfuric acid. This converts the alcohol into a better leaving group (water).
Step 2: Formation of Carbocation
After protonation, the alcohol loses a water molecule, leading to the formation of a carbocation. The stability of this carbocation is crucial in determining whether rearrangement is favorable.
Step 3: Analyzing Carbocation Stability
- Option 1: If the carbocation formed is a primary carbocation, it is less stable and unlikely to rearrange.
- Option 2: If the carbocation is secondary or tertiary, it can rearrange to form a more stable carbocation (e.g., from secondary to tertiary).
- Option 3: If the carbocation is part of a cyclic structure, it may undergo ring expansion or rearrangement to achieve a more stable configuration.
Step 4: Identify the Correct Option
Based on the analysis of the carbocations formed in each option:
- In Option 1, the carbocation formed is primary and does not favor rearrangement due to its instability.
- In Option 2, the carbocation can rearrange to a more stable tertiary carbocation.
- In Option 3, the carbocation can also rearrange due to ring expansion.
- In Option 4, the carbocation formed is secondary and can rearrange to a more stable tertiary carbocation.
Conclusion
Thus, the option where dehydration by concentrated H2SO4 does not favor rearrangement is Option 1.
---
The first step in the dehydration mechanism is the protonation of the alcohol by concentrated sulfuric acid. This converts the alcohol into a better leaving group (water).
After protonation, the alcohol loses a water molecule, leading to the formation of a carbocation. The stability of this carbocation is crucial in determining whether rearrangement is favorable.
- Option 1: If the carbocation formed is a primary carbocation, it is less stable and unlikely to rearrange.
- Option 2: If the carbocation is secondary or tertiary, it can rearrange to form a more stable carbocation (e.g., from secondary to tertiary).
- Option 3: If the carbocation is part of a cyclic structure, it may undergo ring expansion or rearrangement to achieve a more stable configuration.
Based on the analysis of the carbocations formed in each option:
- In Option 1, the carbocation formed is primary and does not favor rearrangement due to its instability.
- In Option 2, the carbocation can rearrange to a more stable tertiary carbocation.
- In Option 3, the carbocation can also rearrange due to ring expansion.
- In Option 4, the carbocation formed is secondary and can rearrange to a more stable tertiary carbocation.
Thus, the option where dehydration by concentrated H2SO4 does not favor rearrangement is Option 1.
Similar Questions
Knowledge Check
Conc. H2SO4 is not
AHygroscopicBDehydrating agentCSulphonating agentDefflorescentWhich one of the following reacts with conc. H2SO4 ?
AAuBAgCPtDPbWhich of the following gases can be dried by conc. H2SO4
AHClBHBrCHIDH2S
Which one of the following reacts with conc. H2SO4?
View SolutionWhich of the following gases can be dried by conc. H2SO4 ?
View SolutionWhich of the following gases can be dried by conc. H2SO4 ?
View SolutionWhich one of the following does not dissolve in conc. H2SO4?
View SolutionWhich of the following gases cannot be dried by conc. H2SO4?
View Solution
