In Friedel-Craft's reaction, toulene can be prepared by
C6H6+CH3Cl
C6H6+CH4
C6H6+CH2Cl2
C6H6+CH3COCl
The correct Answer is:1
To prepare toluene via Friedel-Crafts reaction, we can follow these steps:
1. Identify the Reactants:
- The primary reactants for the Friedel-Crafts alkylation are benzene (C6H6) and an alkyl halide (R-X), where R represents an alkyl group and X is a halogen.
2. Choose the Lewis Acid Catalyst:
- A Lewis acid, such as aluminum chloride (AlCl3), is required to facilitate the reaction. The Lewis acid will help in generating the electrophile from the alkyl halide.
3. Formation of the Electrophile:
- When the alkyl halide (R-X) reacts with the Lewis acid (AlCl3), it generates a carbocation (R^+) or an electrophile. For example, if we use methyl chloride (CH3Cl), the reaction will produce a methyl cation (CH3^+).
4. Electrophilic Attack on Benzene:
- The generated electrophile (R^+) will then attack the benzene ring. This occurs at one of the carbon atoms in the benzene, resulting in the formation of a sigma complex (also known as an arenium ion).
5. Deprotonation:
- The sigma complex is unstable and will lose a proton (H^+) to restore the aromaticity of the benzene ring. This step results in the formation of toluene (C7H8).
6. Final Product:
- The final product of the reaction is toluene, which has a methyl group (CH3) attached to the benzene ring.
Summary of the Reaction:
Benzene + Methyl chloride (CH3Cl) + AlCl3 → Toluene (C7H8) + HCl
- The primary reactants for the Friedel-Crafts alkylation are benzene (C6H6) and an alkyl halide (R-X), where R represents an alkyl group and X is a halogen.
- A Lewis acid, such as aluminum chloride (AlCl3), is required to facilitate the reaction. The Lewis acid will help in generating the electrophile from the alkyl halide.
- When the alkyl halide (R-X) reacts with the Lewis acid (AlCl3), it generates a carbocation (R^+) or an electrophile. For example, if we use methyl chloride (CH3Cl), the reaction will produce a methyl cation (CH3^+).
- The generated electrophile (R^+) will then attack the benzene ring. This occurs at one of the carbon atoms in the benzene, resulting in the formation of a sigma complex (also known as an arenium ion).
- The sigma complex is unstable and will lose a proton (H^+) to restore the aromaticity of the benzene ring. This step results in the formation of toluene (C7H8).
- The final product of the reaction is toluene, which has a methyl group (CH3) attached to the benzene ring.
Benzene + Methyl chloride (CH3Cl) + AlCl3 → Toluene (C7H8) + HCl
Topper's Solved these Questions
- HYDROCARBONSBook:AAKASH INSTITUTEChapter:HYDROCARBONSExercise:Assignment(Section - D) (Assertion -Reason Type Question)
Explore 24 Videos
- HYDROCARBONSBook:AAKASH INSTITUTEChapter:HYDROCARBONSExercise:Assignment(Section - B) (Obejctive type question)
Explore 13 Videos
- HALOALKANES AND HALOARENES Book:AAKASH INSTITUTEChapter:HALOALKANES AND HALOARENES Exercise:ASSIGNMENT SECTION -D
Explore 15 Videos
- HYDROGENBook:AAKASH INSTITUTEChapter:HYDROGENExercise:ASSIGNMENT (SECTION - D) (Assertion-Reason Type question)
Explore 15 Videos
Similar Questions
Knowledge Check
In Friedal craft reaction Toluene can be prepared by:
AC6H6+CH3CIBC6H5CI+CH4CC6H6CH2CI2DC6H6+CH3COCIFriedel-Crafts acylation is-
Aα-acylation of a carbonyl compoundBacylation of phenols to generate estersCacylation of aliphatic olefinsDacylation of aromatic nucleusFriedel-Crafts reaction is not given by:
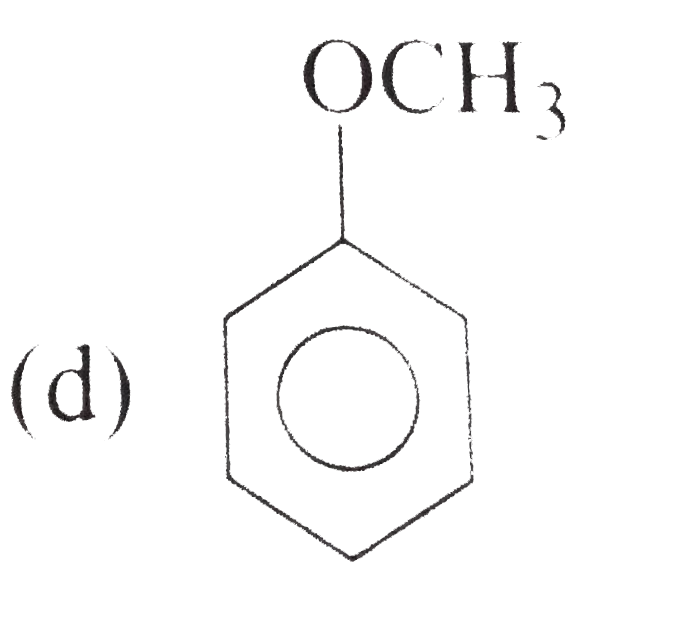
A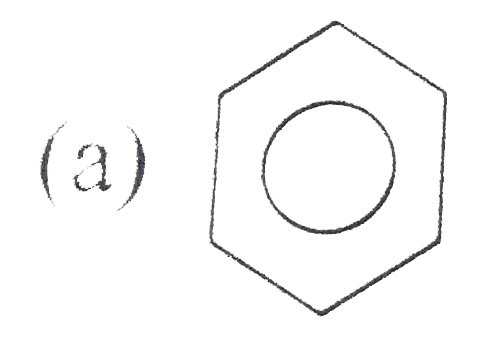 B
B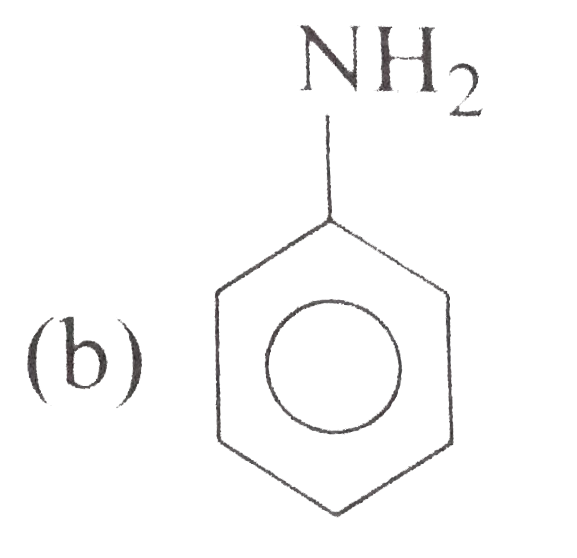 C
C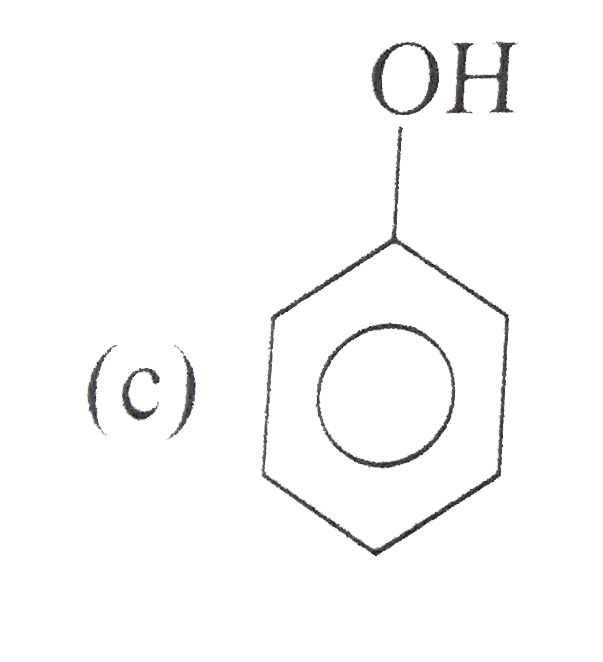 D
D