A : has 12 πeelectrons i.e. 4n, πe electrons.
R : It is an antiaromatic compound.
If both Assertion & Reason are true and the reason is the correct explanation of the assertion, then mark (1).
If both Assertion & Reason are true but the reason is not the correct explanation of the assertion, then mark (2)
if Assertion is true statement but Reason is false, then mark (3)
If both Assertion and Reason are false statements, then mark (4)
The correct Answer is:3
Step by step video & image solution for A : has 12 pieelectrons i.e. 4n, pie electrons. R : It is an antiaromatic compound. by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- HYDROCARBONSBook:AAKASH INSTITUTEChapter:HYDROCARBONSExercise:Assignment(Section - C) (Previous Years Questions)
Explore 69 Videos
- HALOALKANES AND HALOARENES Book:AAKASH INSTITUTEChapter:HALOALKANES AND HALOARENES Exercise:ASSIGNMENT SECTION -D
Explore 15 Videos
- HYDROGENBook:AAKASH INSTITUTEChapter:HYDROGENExercise:ASSIGNMENT (SECTION - D) (Assertion-Reason Type question)
Explore 15 Videos
Similar Questions
H3C−C≡CHH2O,H4SO4−−−−−−−→HgSO4intermediateA→productB
View SolutionIn the following questions, a statement of assertion (A) is following by a statement of reason (R )
A : when chloroform is obtained from bleaching powder and ethanol then chlorine acts as oxidising agent only.
R : It oxidises ethanol into acetic acid.
View SolutionA : ScH2 is an example of ionic hydride.
R : All metal forms ionic hydride.
View SolutionHow does electron deficient compound BF_(3) achieve electron saturation, i.e. fully occupied outer electronic shells?
View SolutionChoose the compounds below that are antiaromatic :
View SolutionAssertion (A) The compound tetraene has the following structural formul.

It is cyclic and has conjugated 8π-electron system but it is not an aromatic compound.
Reason (R ) (4n+2)π electrons rule does not hold good and ring is not planar.
View SolutionAmong the following,
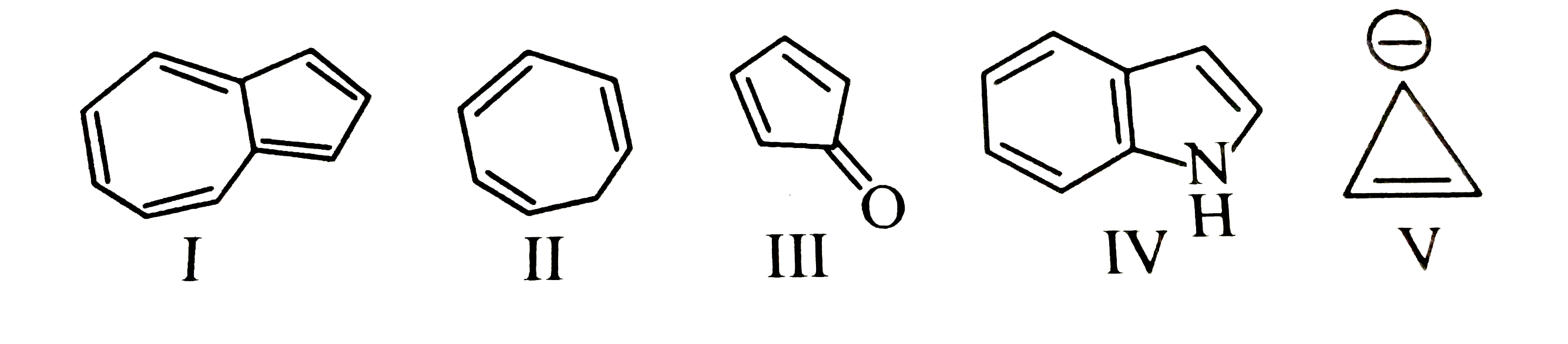
the antiaromatic compounds are
View SolutionHow does electron deficient compound BF_(3) achieve electronic saturation i.e., fully occupied outer electron shells ?
View SolutionFor a compound to be aromatic it must have (4n + 2) pi electrons.
View Solutionनिम्न में से कौन-सा पाई-बन्धित कार्ब-धात्विक यौगिक नहीं है?
View SolutionWhich of the following is an antiaromatic compound ?
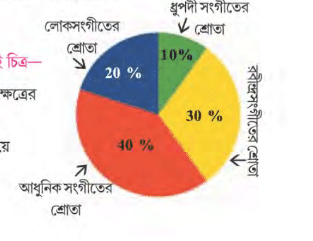
View Solutionনীচের পাই চিত্র দেখো ও প্রশ্নের উত্তর দাও - শ্রোতারা কোন কোন ধরনের গান পছন্দ করেন তার পাই চিত্র দেওয়া হলো- পাই চিত্র থেকে কোন ধরনের গানের শ্রোতা সবচেয়ে বেশি ?
View SolutionAssertion (A) The compound tetraene has the following structural formula.

It is cyclic and has conjugated 8π-electron system but it is not an aromatic compound.
Reason (R ) (4n+2)π electrons rule does not hold good and ring is not planar.
View Solutionনীচের পাই চিত্র দেখো ও প্রশ্নের উত্তর দাও - শ্রোতারা কোন কোন ধরনের গান পছন্দ করেন তার পাই চিত্র দেওয়া হলো- পাই চিত্র থেকে কোন ধরনের গানের শ্রোতা সবচেয়ে বেশি ?

View SolutionAntiAromatic |Non Aromatic Compounds
View SolutionAbove given pie chart is showing number of vertebrate species. In above pie chart identify 'R' is representing species of:-
View Solution
