Out of phenol and benzene, which can be more easily nitrated ?
Phenol can be more easily nitrated than benzene. This is because, -OH group is electron releasing group and it increases electron density on the benzene ring, particularly at the ortho and para positions. Since nitration involves attack of nitronium (NO+2) ion, its attack on phenol will be easier than on benzene where no electron releasing group is present
Similar Questions
Nitration is an example of aromatic electrophilic substitution and its rate depends upon the group already present in the benzene ring. Out of benzene and phenol, which one is more easily nitrated and why ?
View SolutionNitration is an example of aromatic electrophilic substitution and its rate depends upon the group already present in the benzene ring. Out of benzene and phenol, which one is more easily nitrated and why ?
View Solution
Knowledge Check
Which of the following will be easily nitrated?
A B
B CCH3NO2DC6H5NO2
CCH3NO2DC6H5NO2Which of the following will be easily nitrated
A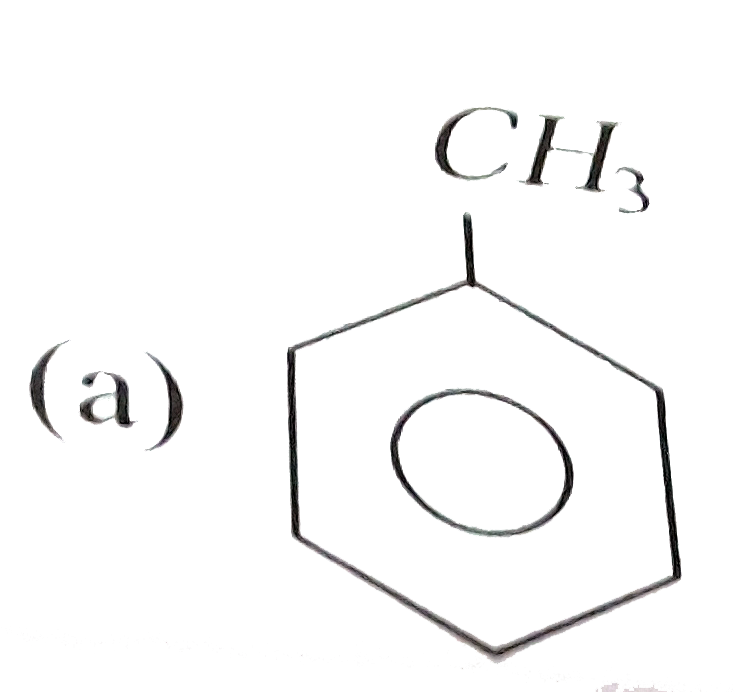 B
B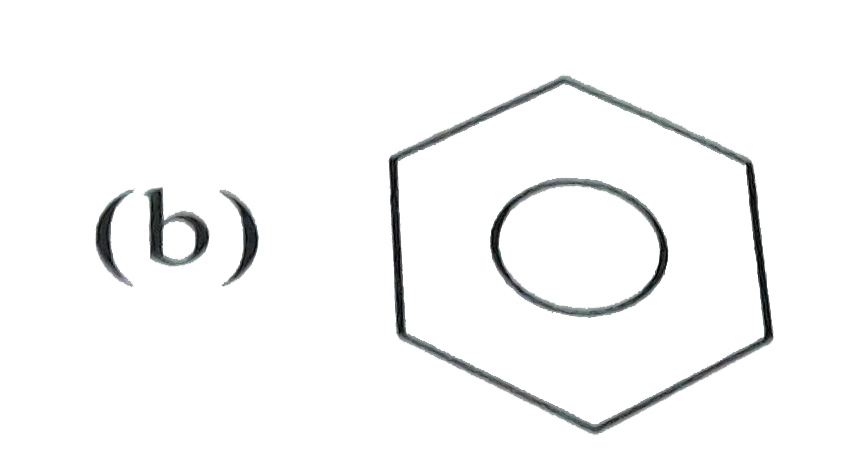 CCH3NO2DC6H5NO2
CCH3NO2DC6H5NO2In the following question, out of the four alternatives, select the alternative which is the best substitute of the phrase.
That which can be easily believedAAssuranceBCredibleCObviousDHonorary
Nitration is an example of electrophilic substitution and its rate depends upon the group already present in the benzene ring. Out of benzene and phenol, which one is more easily nitrated and why?
View SolutionPhenol to Benzene
View SolutionOut of benzene and phenol which is more easily nitrated and why?
View SolutionOut of phenol and phenate ion, which is more resonance stabilised?
View SolutionOut of vinyl chloride and ethyl chloride which is more easily hydrolysed?
View Solution

