Cannizaro's reaction is not given by ________
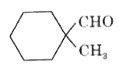
HCHO
CH3CHO
The correct Answer is:D
To determine which compound does not undergo Cannizzaro's reaction, we need to analyze the options based on the presence of alpha hydrogen atoms. Cannizzaro's reaction occurs with aldehydes that lack alpha hydrogens. Here’s a step-by-step solution:
Step 1: Understand Cannizzaro's Reaction
Cannizzaro's reaction is a redox reaction that occurs in aldehydes that do not have alpha hydrogen atoms. In this reaction, one molecule of the aldehyde is oxidized to a carboxylic acid, while another molecule is reduced to an alcohol.
Step 2: Analyze Each Option
We will analyze each option to check for the presence of alpha hydrogen atoms.
Option A: Aldehyde with a six-membered ring (e.g., cyclohexanecarbaldehyde)
- Structure: CHO (aldehyde) attached to a six-membered ring.
- Alpha Carbon: The carbon next to the aldehyde carbon is part of the ring and has no hydrogen atoms.
- Conclusion: No alpha hydrogen present; this compound can undergo Cannizzaro's reaction.
Option B: Aldehyde with a carbon chain (e.g., 2-methylbutanal)
- Structure: CH3-CHO (aldehyde) with a carbon chain.
- Alpha Carbon: The carbon next to the aldehyde carbon has three hydrogen atoms.
- Conclusion: Alpha hydrogen present; this compound cannot undergo Cannizzaro's reaction.
Option C: Aldehyde with no alpha carbon (e.g., formaldehyde)
- Structure: HCHO (formaldehyde).
- Alpha Carbon: There is no carbon adjacent to the aldehyde carbon.
- Conclusion: No alpha hydrogen present; this compound can undergo Cannizzaro's reaction.
Option D: Acetaldehyde (ethanal)
- Structure: CH3CHO.
- Alpha Carbon: The carbon next to the aldehyde has three hydrogen atoms.
- Conclusion: Alpha hydrogen present; this compound cannot undergo Cannizzaro's reaction.
Step 3: Identify the Correct Answer
Based on the analysis:
- The compound that does not undergo Cannizzaro's reaction is Option B (the aldehyde with a carbon chain that has alpha hydrogen).
Final Answer
Cannizzaro's reaction is not given by Option B.
Cannizzaro's reaction is a redox reaction that occurs in aldehydes that do not have alpha hydrogen atoms. In this reaction, one molecule of the aldehyde is oxidized to a carboxylic acid, while another molecule is reduced to an alcohol.
We will analyze each option to check for the presence of alpha hydrogen atoms.
- Structure: CHO (aldehyde) attached to a six-membered ring.
- Alpha Carbon: The carbon next to the aldehyde carbon is part of the ring and has no hydrogen atoms.
- Conclusion: No alpha hydrogen present; this compound can undergo Cannizzaro's reaction.
- Structure: CH3-CHO (aldehyde) with a carbon chain.
- Alpha Carbon: The carbon next to the aldehyde carbon has three hydrogen atoms.
- Conclusion: Alpha hydrogen present; this compound cannot undergo Cannizzaro's reaction.
- Structure: HCHO (formaldehyde).
- Alpha Carbon: There is no carbon adjacent to the aldehyde carbon.
- Conclusion: No alpha hydrogen present; this compound can undergo Cannizzaro's reaction.
- Structure: CH3CHO.
- Alpha Carbon: The carbon next to the aldehyde has three hydrogen atoms.
- Conclusion: Alpha hydrogen present; this compound cannot undergo Cannizzaro's reaction.
Based on the analysis:
- The compound that does not undergo Cannizzaro's reaction is Option B (the aldehyde with a carbon chain that has alpha hydrogen).
Cannizzaro's reaction is not given by Option B.
Topper's Solved these Questions
- ALDEHYDES ,KETONES AND CARBOXYLIC ACIDSBOOK - MODERN PUBLICATIONCHAPTER - ALDEHYDES ,KETONES AND CARBOXYLIC ACIDSEXERCISE - NCERT FILE (NCERT EXEMPLAR PROBLEMS ) (MULTIPLE CHOICE QUESTIONS ( TYPE -II)
6 Videos
- ALDEHYDES ,KETONES AND CARBOXYLIC ACIDSBOOK - MODERN PUBLICATIONCHAPTER - ALDEHYDES ,KETONES AND CARBOXYLIC ACIDSEXERCISE - NCERT FILE (NCERT EXEMPLAR PROBLEMS ) (SHORT ANSWER TYPE QUESTIONS )
19 Videos
- ALDEHYDES ,KETONES AND CARBOXYLIC ACIDSBOOK - MODERN PUBLICATIONCHAPTER - ALDEHYDES ,KETONES AND CARBOXYLIC ACIDSEXERCISE - NCERT FILE (NCERT TEXTBOOK EXERCISES )
20 Videos
- ALCOHOLS, PHENOLS AND ETHERSBOOK - MODERN PUBLICATIONCHAPTER - ALCOHOLS, PHENOLS AND ETHERSEXERCISE - UNIT PRACTICE TEST (FOR BOARD EXAMINATION)
12 Videos
- BIOMOLECULESBOOK - MODERN PUBLICATIONCHAPTER - BIOMOLECULESEXERCISE - UNIT PRACTICE TEST FOR BOARD EXAMINATION
13 Videos
Similar Questions
(a) What is Cannizaro's reaction ? Write its mechanism.
(b) Discuss the structure of carbonyl group.
View SolutionAn organic compound with the molecular folmula C9H10O form 2,4-DNP derivative, reduces Tollens reagent, and undergoes Cannizaro reaction. On vigorous oxidation, it gives 1,2-benzenedicarboxylic acid. Identify the compound.
View Solution
Knowledge Check
Cannizzaro reaction is not given by
AmethanalBphenyl methanalC2, 2-dimethylbutanalDphenyl ethanal.The Cannizaro reaction is not given by
ATrimethyl acetaldehydeBAcetaldehydeCBenzaldehydeDFormaldehydeThe Cannizzaro reaction is not given by
AtrimethylacetaldehydeBacetaldehydeCbenzaldehydeDformaldehyde.
Cannizaro's reaction is given by
View SolutionCannizaro reaction is preformed by
View SolutionCannizaro reaction is performed by
View SolutionCannizaro reaction is performed by
View SolutionCannizaro's reaction is given by only those compounds in which α-H atoms are absent , CH3CHO , due to the pressure of α-H atoms , does not give Cannizaro's reactions.
View Solution

