Why should one always use purest monomer in free radical polymerisation reaction ?
Step-by-Step Solution:
1. Understanding Free Radical Polymerization:
- Free radical polymerization is a process where free radicals initiate the polymerization of monomers to form long-chain polymers.
- A free radical is a highly reactive species with an unpaired electron.
2. Role of Monomers:
- Monomers are the building blocks of polymers. In free radical polymerization, the purity of the monomer is crucial for the success of the reaction.
3. Impurities in Monomers:
- If the monomer is not pure, it may contain impurities that can interfere with the polymerization process.
- These impurities can react with the free radicals formed during the initiation step.
4. Impact of Impurities on the Reaction:
- When impurities react with free radicals, they can terminate the growing polymer chains prematurely or create unwanted side reactions.
- This leads to a decrease in the overall efficiency of the polymerization process, resulting in lower yields of the desired polymer.
5. Chain Propagation and Termination:
- In a successful polymerization, the free radicals should propagate the chain by reacting with the pure monomer.
- If impurities are present, they can compete with the monomer for the free radicals, slowing down the reaction and preventing the formation of the desired polymer.
6. Conclusion:
- Therefore, using the purest monomer ensures that the free radicals can effectively propagate the polymer chain without interference from impurities, leading to a higher yield and better quality of the final polymer product.
- Free radical polymerization is a process where free radicals initiate the polymerization of monomers to form long-chain polymers.
- A free radical is a highly reactive species with an unpaired electron.
- Monomers are the building blocks of polymers. In free radical polymerization, the purity of the monomer is crucial for the success of the reaction.
- If the monomer is not pure, it may contain impurities that can interfere with the polymerization process.
- These impurities can react with the free radicals formed during the initiation step.
- When impurities react with free radicals, they can terminate the growing polymer chains prematurely or create unwanted side reactions.
- This leads to a decrease in the overall efficiency of the polymerization process, resulting in lower yields of the desired polymer.
- In a successful polymerization, the free radicals should propagate the chain by reacting with the pure monomer.
- If impurities are present, they can compete with the monomer for the free radicals, slowing down the reaction and preventing the formation of the desired polymer.
- Therefore, using the purest monomer ensures that the free radicals can effectively propagate the polymer chain without interference from impurities, leading to a higher yield and better quality of the final polymer product.
Topper's Solved these Questions
- POLYMERSBOOK - MODERN PUBLICATIONCHAPTER - POLYMERSEXERCISE - Competition file (OBJECTIVE TYPE QUESTIONS) (A. MULTIPLE CHOICE QUESTIONS)(Polymers and their Classifications)
10 Videos
- POLYMERSBOOK - MODERN PUBLICATIONCHAPTER - POLYMERSEXERCISE - Competition file (OBJECTIVE TYPE QUESTIONS) (A. MULTIPLE CHOICE QUESTIONS)(Some Important Polymers)
15 Videos
- POLYMERSBOOK - MODERN PUBLICATIONCHAPTER - POLYMERSEXERCISE - Revision Exercises (Objective Questions)(Short Answer Questions)
46 Videos
- ORGANIC COMPOUNDS CONTAINING NITROGEN BOOK - MODERN PUBLICATIONCHAPTER - ORGANIC COMPOUNDS CONTAINING NITROGEN EXERCISE - UNIT PRACTICE TEST
5 Videos
- SOLID STATEBOOK - MODERN PUBLICATIONCHAPTER - SOLID STATEEXERCISE - UNIT PRACTICE TEST
13 Videos
Similar Questions
Which of the following is naturally occurring polymer?
View SolutionNeoprene is a polymer of
View SolutionWhat are elastomers ? Write the chemical equation to represent the preparation of Buna-S
View SolutionConvert
(i) 3-Methylaniline into 3-nitrotoluene.
(ii) Aniline into 1,3,5 - tribromobenzene.
View SolutionThe ratio of closed packed atoms to tetrahedral holes in cubic close packing is :
View Solution(a)Why is the purest monomer used in free radical polymerisation?
(b).How does the presence ofCl4orCBr4influence the course of vinylic free radical polymersation.
(c )what is the monomer of the polymer given below:
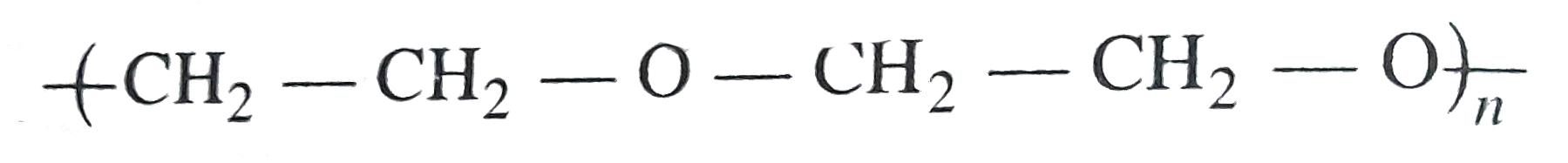
(d)Whydoesho≤develope∈nylons→ck∈gwhenadropofHClis added to it?
View SolutionFree radical polymerisation requires a free radical initior.The most commonly used free radical initiator is:
View SolutionWhy should the monomer used in addition polymerisation through free radical pathway be very pure?
View Solutionमुक्त मूलक बहुलकन में हमेशा शुद्धतम् एकलक का प्रयोग क्यों करना चाहिए ?
View SolutionThe monomers used in addition polymerisation through free radical should be every pure because
View SolutionWhy is It important to have monomers ln their purest form In free radical addition polymerisation?
View SolutionWhy should the monomers used in addition polymerisation through free radical pathway be very pure?
View SolutionWhy should the monomers used in addition polymerisation through free radical pathway by very pure ?
View Solutionमुक्त मूलक बहुलीकरण में हमेशा शुद्धतम् एकलक का प्रयोग करना चाहिए, क्यों?
View SolutionThe free radical polymerisation of the monomer 2 - chloro buta - 1,3-diene gives
View Solution

