Match list I with list II and select the correct answer using the codes given below:
List I (Polymers)List II (Monomers)1. Buna - NA. Phthalic acid and ethylene glycol2. Nylon -6, 6B. Terephthalic acid and ethylene glycol3. DacronC. Hxamethylenediamine and adipic acid4. Glyptal plasticD. Isobutylene and isopreneE. Acrylonitrile and butadiene
1−B,2−A,3−D,4−E
1−C,2−D,3−A,4−B
1−D,2−C,3−B,4−A
1−E,2−C,3−B,4−A
To solve the question of matching the polymers in List I with their corresponding monomers in List II, we will analyze each polymer and identify its monomer components step by step.
1. Identify the Monomers for Buna-N:
- Buna-N is a copolymer made from two monomers: acrylonitrile and butadiene.
- Match: Buna-N → E (Acrylonitrile and butadiene).
2. Identify the Monomers for Nylon-6,6:
- Nylon-6,6 is formed from hexamethylenediamine and adipic acid through condensation polymerization.
- Match: Nylon-6,6 → C (Hexamethylenediamine and adipic acid).
3. Identify the Monomers for Dacron:
- Dacron is a polyester made from terephthalic acid and ethylene glycol.
- Match: Dacron → B (Terephthalic acid and ethylene glycol).
4. Identify the Monomers for Glyptal Plastic:
- Glyptal is produced from phthalic acid and ethylene glycol.
- Match: Glyptal plastic → A (Phthalic acid and ethylene glycol).
Final Matches:
- 1. Buna-N → E
- 2. Nylon-6,6 → C
- 3. Dacron → B
- 4. Glyptal plastic → A
Summary of Matches:
- 1 → E
- 2 → C
- 3 → B
- 4 → A
- Buna-N is a copolymer made from two monomers: acrylonitrile and butadiene.
- Match: Buna-N → E (Acrylonitrile and butadiene).
- Nylon-6,6 is formed from hexamethylenediamine and adipic acid through condensation polymerization.
- Match: Nylon-6,6 → C (Hexamethylenediamine and adipic acid).
- Dacron is a polyester made from terephthalic acid and ethylene glycol.
- Match: Dacron → B (Terephthalic acid and ethylene glycol).
- Glyptal is produced from phthalic acid and ethylene glycol.
- Match: Glyptal plastic → A (Phthalic acid and ethylene glycol).
- 1. Buna-N → E
- 2. Nylon-6,6 → C
- 3. Dacron → B
- 4. Glyptal plastic → A
- 1 → E
- 2 → C
- 3 → B
- 4 → A
Similar Questions
Match List I with List II and select the correct answer using the codes given below the lists :
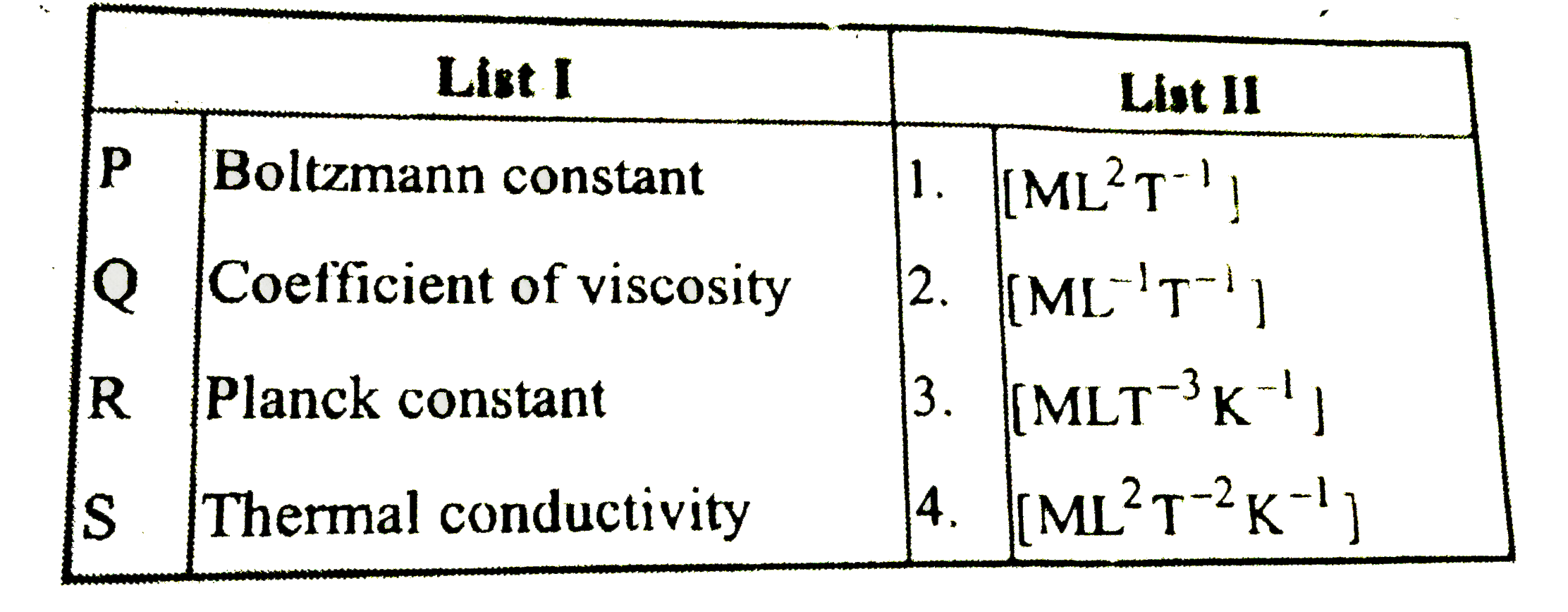
View SolutionMatch List - I with List II and select the correct answer using the codes given below the lists

View SolutionMatch List I with List II and select the correct answer using the code given below the Lists :
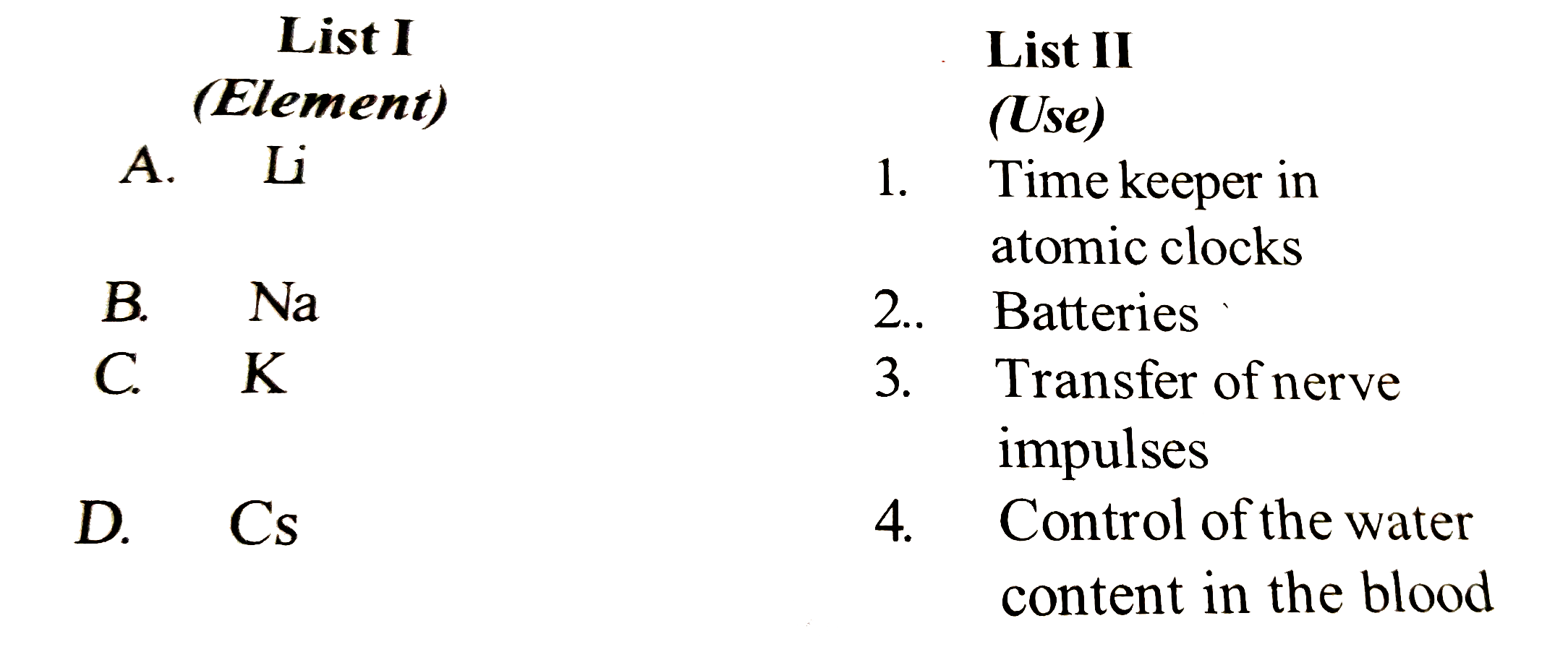
View SolutionMatch List I with List II and select the correct answer using the code given below the Lists:

View SolutionMatch List I with List II and select the correct answer using the code given below the Lists:

View SolutionMatch List I with List II and select the correct answer using the code given below the Lists :

View SolutionMatch List I with List II and select the correct answer using the code given below the Lists:

View SolutionMatch List I with List II and select the correct answer using the code given below the Lists :

View SolutionMatch List I with List II and select the correct answer using the code given below the Lists:

View SolutionMatch List I with List II and select the correct answer using the code given below the Lists:

View Solution
